当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rh-Catalyzed Coupling Reactions of Fluoroalkyl N-Sulfonylhydrazones with Azides Leading to α-Trifluoroethylated Imines
Organic Letters ( IF 5.2 ) Pub Date : 2022-11-29 , DOI: 10.1021/acs.orglett.2c03773 Zhongxue Fang 1 , Yanmei Gong 1 , Binbin Liu 2 , Jin Zhang 2 , Xinyue Han 2 , Zhaohong Liu 2 , Yongquan Ning 2
Organic Letters ( IF 5.2 ) Pub Date : 2022-11-29 , DOI: 10.1021/acs.orglett.2c03773 Zhongxue Fang 1 , Yanmei Gong 1 , Binbin Liu 2 , Jin Zhang 2 , Xinyue Han 2 , Zhaohong Liu 2 , Yongquan Ning 2
Affiliation
|
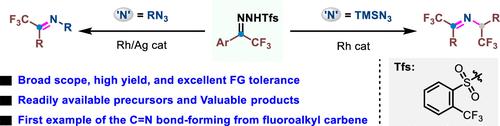
Herein we describe the pioneering Rh-catalyzed coupling reactions of a fluoroalkyl carbene with azides to access α-trifluoroethylated imines, where fluoroalkyl N-sulfonylhydrazones are used as fluoroalkyl diazo surrogates. Remarkably, using TMSN3 as the N source, two C–N bond formation products were obtained. Furthermore, the α-trifluoroethylated imine products could be easily reduced to the corresponding N-trifluoroethylated anilines. Experimental results and theoretical calculations justify a stepwise reaction pathway involving the formation of rhodium carbene, the addition of HN3, and C═N bond formation.
中文翻译:

氟烷基 N-磺酰腙与叠氮化物生成 α-三氟乙基化亚胺的 Rh 催化偶联反应
在此,我们描述了氟代烷基卡宾与叠氮化物的开创性 Rh 催化偶联反应以获得 α-三氟乙基化亚胺,其中氟代烷基N-磺酰腙用作氟代烷基重氮替代物。值得注意的是,使用 TMSN 3作为 N 源,获得了两种 C-N 键形成产物。此外,α-三氟乙基化亚胺产物可以很容易地还原成相应的N-三氟乙基化苯胺。实验结果和理论计算证明了涉及铑卡宾形成、HN 3加成和C=N键形成的逐步反应途径。
更新日期:2022-11-29
中文翻译:

氟烷基 N-磺酰腙与叠氮化物生成 α-三氟乙基化亚胺的 Rh 催化偶联反应
在此,我们描述了氟代烷基卡宾与叠氮化物的开创性 Rh 催化偶联反应以获得 α-三氟乙基化亚胺,其中氟代烷基N-磺酰腙用作氟代烷基重氮替代物。值得注意的是,使用 TMSN 3作为 N 源,获得了两种 C-N 键形成产物。此外,α-三氟乙基化亚胺产物可以很容易地还原成相应的N-三氟乙基化苯胺。实验结果和理论计算证明了涉及铑卡宾形成、HN 3加成和C=N键形成的逐步反应途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号