当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Heterogeneous molecular Co–N–C catalysts for efficient electrochemical H2O2 synthesis
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2022-11-29 , DOI: 10.1039/d2ee02734h Chang Liu 1 , Zixun Yu 1, 2 , Fangxin She 1 , Jiaxiang Chen 1 , Fangzhou Liu 1 , Jiangtao Qu 3 , Julie M. Cairney 3 , Chongchong Wu 4 , Kailong Liu 5 , Weijie Yang 5 , Huiling Zheng 6 , Yuan Chen 1 , Hao Li 2 , Li Wei 1
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2022-11-29 , DOI: 10.1039/d2ee02734h Chang Liu 1 , Zixun Yu 1, 2 , Fangxin She 1 , Jiaxiang Chen 1 , Fangzhou Liu 1 , Jiangtao Qu 3 , Julie M. Cairney 3 , Chongchong Wu 4 , Kailong Liu 5 , Weijie Yang 5 , Huiling Zheng 6 , Yuan Chen 1 , Hao Li 2 , Li Wei 1
Affiliation
|
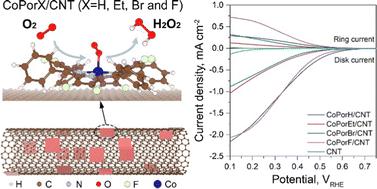
Sustainable hydrogen peroxide can be produced via the oxygen reduction reaction catalyzed by single-atom cobalt–nitrogen–carbon catalysts. However, the precise catalyst atomic structure tailoring remains difficult, limiting the a priori design and activity improvement. We address this limitation by constructing heterogeneous molecular catalysts from cobalt porphyrins adsorbed on a carbon nanotube substrate. Based on the explicit atomistic models, our first-principle calculation suggested that porphyrin β-substituents and the carbon substrate can synergistically modulate Co properties and catalytic activity. An octafluoro-substituted catalyst was predicted as optimal and further validated by experiments, exhibiting >94% H2O2 selectivity and a high turnover frequency of 3.51 per second at an overpotential of 200 millivolts in an acid electrolyte. It can reach a maximum H2O2 productivity of 10.76 molH2O2 gcat−1 h−1 in a two-electrode electrolyzer, delivering pure H2O2 solutions that can be used directly for water treatment and chemical production.
中文翻译:

用于高效电化学 H2O2 合成的多相分子 Co-N-C 催化剂
可持续的过氧化氢可以通过单原子钴-氮-碳催化剂催化的氧还原反应生产。然而,精确的催化剂原子结构调整仍然很困难,限制了先验设计和活性的提高。我们通过从吸附在碳纳米管基板上的钴卟啉构建多相分子催化剂来解决这一限制。基于显式原子模型,我们的第一性原理计算表明卟啉 β-取代基和碳底物可以协同调节钴的性质和催化活性。八氟取代催化剂被预测为最佳催化剂,并通过实验进一步验证,表现出 >94% H 2 O 2在酸性电解质中,在 200 毫伏的过电势下,选择性和每秒 3.51 次的高转换频率。它可以在双电极电解槽中达到10.76 mol H 2 O 2 g cat −1 h −1的最大 H 2 O 2生产率,提供可直接用于水处理和化学生产的纯 H 2 O 2溶液。
更新日期:2022-11-30
中文翻译:

用于高效电化学 H2O2 合成的多相分子 Co-N-C 催化剂
可持续的过氧化氢可以通过单原子钴-氮-碳催化剂催化的氧还原反应生产。然而,精确的催化剂原子结构调整仍然很困难,限制了先验设计和活性的提高。我们通过从吸附在碳纳米管基板上的钴卟啉构建多相分子催化剂来解决这一限制。基于显式原子模型,我们的第一性原理计算表明卟啉 β-取代基和碳底物可以协同调节钴的性质和催化活性。八氟取代催化剂被预测为最佳催化剂,并通过实验进一步验证,表现出 >94% H 2 O 2在酸性电解质中,在 200 毫伏的过电势下,选择性和每秒 3.51 次的高转换频率。它可以在双电极电解槽中达到10.76 mol H 2 O 2 g cat −1 h −1的最大 H 2 O 2生产率,提供可直接用于水处理和化学生产的纯 H 2 O 2溶液。



























 京公网安备 11010802027423号
京公网安备 11010802027423号