Analytica Chimica Acta ( IF 6.2 ) Pub Date : 2022-11-21 , DOI: 10.1016/j.aca.2022.340648 Rui Zhang 1 , Rui Zhao 1 , Wenhua Ren 1 , Mo Wang 1 , Gaowei Fan 1 , Jie Shi 1 , Yichuan Song 1 , Xiaojie Zhou 1 , Qingtao Wang 2
|
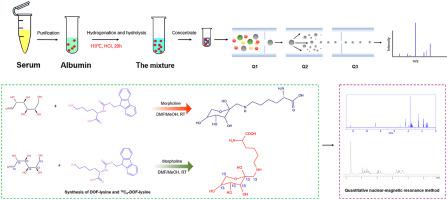
Glycated albumin (GA) in human serum is tested clinically as a short-term indicator for glucose monitoring. Here, we evaluated a candidate serum reference material (RM) at three different GA concentrations to help standardize serum GA measurements. Both albumin and GA were quantitatively determined using isotope-dilution liquid chromatography/tandem mass spectrometry with lysine―4,4,5,5-D4·2HCl (D4-lysine) and Nε-l3C6-(l-deoxy-d-fructose-1-yl)-l-lysine (13C6-DOF-lysine) as internal standards and lysine and synthetic DOF-lysine as calibration standards. The method was evaluated with the RM, JCCRM611-1, from the Reference Material Institute for Clinical Chemistry Standards. The homogeneity and stability of the candidate RMs were examined using a commercial biochemical analyzer. Fifteen units were randomly selected, and statistical analysis showed no inhomogeneity. The candidate RMs were stable for at least 6 months at −80 °C. The coefficients of variation (CVs) for the JCCRM611-1 RM ranged from 3.2% to 2.3%, and the biases ranged from 4.12% to −1.84%. GA was tested at low, medium, and high concentrations, which were quantified as 249.53 ± 13.29, 408.02 ± 11.70, and 637.22 ± 17.03 mmol/mol, respectively. The overall CVs ranged from 0.99% to 2.51%. The candidate RMs can potentially be used to develop a traceability chain to improve the accuracy of GA measurements.
中文翻译:

使用同位素稀释液相色谱/串联质谱法开发基于基质的人糖化白蛋白候选参考物质
人血清中的糖化白蛋白 (GA) 在临床上作为葡萄糖监测的短期指标进行了测试。在这里,我们评估了三种不同 GA 浓度的候选血清参考物质 (RM),以帮助标准化血清 GA 测量。白蛋白和 GA 均使用同位素稀释液相色谱/串联质谱法与赖氨酸―4,4,5,5-D 4 ·2HCl(D 4 -赖氨酸)和 N ε - l3 C 6 -(l-脱氧)定量测定- d -fructose-1-yl)- l -赖氨酸 ( 13 C 6-DOF-赖氨酸)作为内标,赖氨酸和合成 DOF-赖氨酸作为校准标准。使用来自临床化学标准参考材料研究所的 RM JCCRM611-1 对该方法进行了评估。使用商业生化分析仪检查候选 RM 的同质性和稳定性。随机抽取十五个单位,统计分析显示无异质性。候选 RM 在 -80 °C 下至少稳定 6 个月。JCCRM611-1 RM 的变异系数 (CV) 范围为 3.2% 至 2.3%,偏差范围为 4.12% 至 -1.84%。GA 在低、中和高浓度下进行了测试,分别量化为 249.53 ± 13.29、408.02 ± 11.70 和 637.22 ± 17.03 mmol/mol。整体 CV 介于 0.99% 和 2.51% 之间。


























 京公网安备 11010802027423号
京公网安备 11010802027423号