当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lipid Giant Vesicles Engulf Living Bacteria Triggered by Minor Enhancement in Membrane Fluidity
Nano Letters ( IF 10.8 ) Pub Date : 2022-11-28 , DOI: 10.1021/acs.nanolett.2c03475 Shaoying Dai 1, 2 , Xiaoyu Tang 3 , Na Zhang 1, 2 , Haofei Li 1, 2 , Chengzhi He 3 , Yuchun Han 1 , Yilin Wang 1, 2
Nano Letters ( IF 10.8 ) Pub Date : 2022-11-28 , DOI: 10.1021/acs.nanolett.2c03475 Shaoying Dai 1, 2 , Xiaoyu Tang 3 , Na Zhang 1, 2 , Haofei Li 1, 2 , Chengzhi He 3 , Yuchun Han 1 , Yilin Wang 1, 2
Affiliation
|
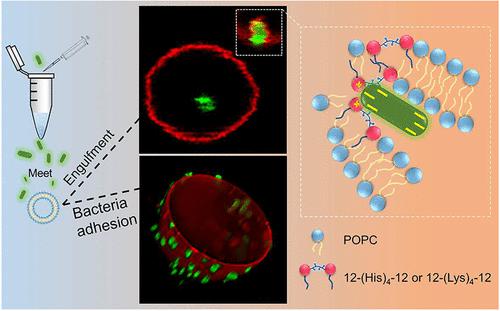
Antibacterial amphiphiles normally kill bacteria by destroying the bacterial membrane. Whether and how antibacterial amphiphiles alter normal cell membrane and lead to subsequent effects on pathogen invasion into cells have been scarcely promulgated. Herein, by taking four antibacterial gemini amphiphiles with different spacer groups to modulate cell-mimic phospholipid giant unilamellar vesicles (GUVs), bacteria adhesion on the modified GUVs surface and bacteria engulfment process by the GUVs are clearly captured by confocal laser scanning microscopy. Further characterization shows that the enhanced cationic surface charge of GUVs by the amphiphiles determines the bacteria adhesion amount, while the involvement of amphiphile in GUVs results in looser molecular arrangement and concomitant higher fluidity in the bilayer membranes, facilitating the bacteria intruding into GUVs. This study sheds new light on the effect of amphiphiles on membrane bilayer and the concurrent effect on pathogen invasion into cell mimics and broadens the nonprotein-mediated endocytosis pathway for live bacteria.
中文翻译:

脂质巨泡吞噬由膜流动性轻微增强引发的活细菌
抗菌两亲物通常通过破坏细菌膜来杀死细菌。抗菌两亲物是否以及如何改变正常细胞膜并导致对病原体侵入细胞的后续影响几乎没有公布。在此,通过采用四种具有不同间隔基团的抗菌双子座两亲物来调节细胞模拟磷脂巨型单层囊泡(GUVs),共聚焦激光扫描显微镜清楚地捕获了修饰后的 GUVs 表面的细菌粘附和 GUVs 的细菌吞噬过程。进一步表征表明,两亲物增强 GUV 的阳离子表面电荷决定了细菌粘附量,而两亲物参与 GUV 导致分子排列更松散,双层膜流动性更高,促进细菌侵入 GUV。这项研究揭示了两亲生物对膜双层的影响以及对病原体侵入细胞模拟物的并发影响,并拓宽了活细菌的非蛋白质介导的内吞途径。
更新日期:2022-11-28
中文翻译:

脂质巨泡吞噬由膜流动性轻微增强引发的活细菌
抗菌两亲物通常通过破坏细菌膜来杀死细菌。抗菌两亲物是否以及如何改变正常细胞膜并导致对病原体侵入细胞的后续影响几乎没有公布。在此,通过采用四种具有不同间隔基团的抗菌双子座两亲物来调节细胞模拟磷脂巨型单层囊泡(GUVs),共聚焦激光扫描显微镜清楚地捕获了修饰后的 GUVs 表面的细菌粘附和 GUVs 的细菌吞噬过程。进一步表征表明,两亲物增强 GUV 的阳离子表面电荷决定了细菌粘附量,而两亲物参与 GUV 导致分子排列更松散,双层膜流动性更高,促进细菌侵入 GUV。这项研究揭示了两亲生物对膜双层的影响以及对病原体侵入细胞模拟物的并发影响,并拓宽了活细菌的非蛋白质介导的内吞途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号