当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Controlling the Size of Au Nanoparticles on Reducible Oxides with the Electrochemical Potential
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-11-28 , DOI: 10.1021/jacs.2c08422 Dongha Kim 1 , Georgios Dimitrakopoulos 1 , Bilge Yildiz 1, 2
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-11-28 , DOI: 10.1021/jacs.2c08422 Dongha Kim 1 , Georgios Dimitrakopoulos 1 , Bilge Yildiz 1, 2
Affiliation
|
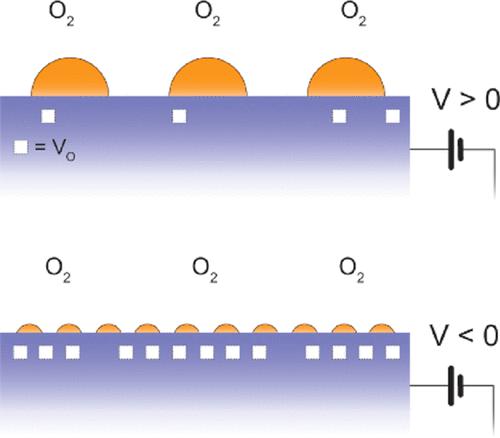
Controlling the size of Au nanoparticles (NPs) and their interaction with the oxide support is important for their catalytic performance in chemical reactions, such as CO oxidation and water-gas shift. It is known that the oxygen vacancies at the surface of support oxides form strong chemical bonding with the Au NPs and inhibit their coarsening and deactivation. The resulting Au/oxygen vacancy interface also acts as an active site for oxidation reactions. Hence, small Au NPs are needed to increase the density of the Au/oxide interface. A dynamic way to control the size of the Au NPs on an oxide support is desirable but has been missing in the field. Here, we demonstrate an electrochemical method to control the size of the Au NPs by controlling the surface oxygen vacancy concentration of the support oxide. Oxides with different reducibilities, La0.8Ca0.2MnO3±δ and Pr0.1Ce0.9O2−δ, are used as model support oxides. By applying the electrochemical potential, we achieve a wide range of effective oxygen pressures, pO2 (10–37–1014 atm), in the support oxides. Applying the cathodic potential creates a high concentration of oxygen vacancies and forms finely distributed Au NPs with sizes of 7–13 nm at 700–770 °C in 10 min, while the anodic potential oxidizes the surface and increases the size of the Au NPs. The onset cathodic potential required to create small Au NPs depends strongly on the reducibility of the support oxide. The Au NPs did not undergo sintering even at 700–770 °C under the cathodic potential and also were stable in catalytically relevant conditions without potential.
中文翻译:

用电化学势控制可还原氧化物上 Au 纳米粒子的尺寸
控制 Au 纳米粒子 (NPs) 的尺寸及其与氧化物载体的相互作用对于它们在化学反应中的催化性能很重要,例如 CO 氧化和水煤气变换。众所周知,载体氧化物表面的氧空位与 Au NPs 形成强烈的化学键合,并抑制它们的粗化和失活。由此产生的 Au/氧空位界面也充当氧化反应的活性位点。因此,需要小的 Au NP 来增加 Au/氧化物界面的密度。需要一种动态的方法来控制氧化物载体上 Au NP 的尺寸,但该领域一直缺少这种方法。在这里,我们展示了一种电化学方法,通过控制载体氧化物的表面氧空位浓度来控制 Au NP 的尺寸。具有不同还原性的氧化物,La0.8 Ca 0.2 MnO 3±δ和 Pr 0.1 Ce 0.9 O 2-δ用作模型支撑氧化物。通过应用电化学势,我们实现了广泛的有效氧气压力 pO 2 (10 –37 –10 14atm), 在载体氧化物中。施加阴极电位会产生高浓度的氧空位,并在 700–770 °C 下 10 分钟内形成尺寸为 7–13 nm 的精细分布的 Au NP,而阳极电位会氧化表面并增加 Au NP 的尺寸。产生小 Au NP 所需的起始阴极电位在很大程度上取决于支撑氧化物的还原性。即使在 700-770 °C 的阴极电位下,Au NPs 也不会发生烧结,并且在没有电位的催化相关条件下也很稳定。
更新日期:2022-11-28
中文翻译:

用电化学势控制可还原氧化物上 Au 纳米粒子的尺寸
控制 Au 纳米粒子 (NPs) 的尺寸及其与氧化物载体的相互作用对于它们在化学反应中的催化性能很重要,例如 CO 氧化和水煤气变换。众所周知,载体氧化物表面的氧空位与 Au NPs 形成强烈的化学键合,并抑制它们的粗化和失活。由此产生的 Au/氧空位界面也充当氧化反应的活性位点。因此,需要小的 Au NP 来增加 Au/氧化物界面的密度。需要一种动态的方法来控制氧化物载体上 Au NP 的尺寸,但该领域一直缺少这种方法。在这里,我们展示了一种电化学方法,通过控制载体氧化物的表面氧空位浓度来控制 Au NP 的尺寸。具有不同还原性的氧化物,La0.8 Ca 0.2 MnO 3±δ和 Pr 0.1 Ce 0.9 O 2-δ用作模型支撑氧化物。通过应用电化学势,我们实现了广泛的有效氧气压力 pO 2 (10 –37 –10 14atm), 在载体氧化物中。施加阴极电位会产生高浓度的氧空位,并在 700–770 °C 下 10 分钟内形成尺寸为 7–13 nm 的精细分布的 Au NP,而阳极电位会氧化表面并增加 Au NP 的尺寸。产生小 Au NP 所需的起始阴极电位在很大程度上取决于支撑氧化物的还原性。即使在 700-770 °C 的阴极电位下,Au NPs 也不会发生烧结,并且在没有电位的催化相关条件下也很稳定。


























 京公网安备 11010802027423号
京公网安备 11010802027423号