当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synergistic effect of oxygen vacancy and dual electrocatalysts in activating anion redox in lithia-based cathodes
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2022-11-28 , DOI: 10.1039/d2ta07674h Ji Hyun Han 1 , Ye Yeong Hwang 1 , Soohyung Park 2 , Jisu Shin 3 , Kyung Joong Yoon 3 , Yun Jung Lee 1
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2022-11-28 , DOI: 10.1039/d2ta07674h Ji Hyun Han 1 , Ye Yeong Hwang 1 , Soohyung Park 2 , Jisu Shin 3 , Kyung Joong Yoon 3 , Yun Jung Lee 1
Affiliation
|
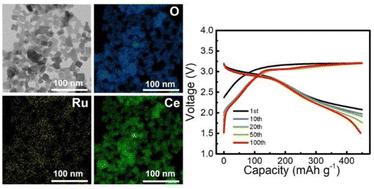
Cathodes operated partially or totally by anion-redox reaction, such as lithium-rich oxides (LROs) or air cathodes of Li–O2 batteries, are attractive owing to their high energy density. Among these, lithia (Li2O)-based cathode systems have drawn attention as an alternative to LROs or Li–O2 battery systems because of their high energy density in safe, sealed environments. However, the practical realization of lithia cathodes is still hindered by poor cycling stability due to the oxygen emission and difficulties in activating the anion-redox reactions during charging. Herein, we employed catalysts that promote both oxygen evolution reaction (OER) and oxygen reduction reaction (ORR) and enhance the oxygen vacancies in the catalysts and lithia to achieve a reversible anion redox. The adopted OER and ORR catalysts were Co3O4 and Ru-doped CeO2, respectively. CeO2 was expected to mediate oxygen through its superior oxygen storage capacity and was thus employed as a complementary catalyst. Oxygen vacancy enhancement was achieved through the heat treatment of Li2O2 producing Li2O2−x, an oxygen-vacancy-rich intermediate phase, and Ru doping into CeO2. Through a comparative investigation, we identified that oxygen vacancies in the cathode considerably enhanced the cycling stability of the lithia cathode. In addition, the Co3O4 OER catalyst is essential for initiating anion redox in lithia; however, the ORR catalyst is required for the reversible operation of this cathode. The synergistic combination of oxygen vacancies, dual OER/ORR catalysts, and the complementary catalyst with oxygen-mediating capability in the Co3O4/Ru–CeO2/Li2O2−x electrodes resulted in superior electrochemical characteristics with cycling stability at a capacity limit of 450 mA h g−1 over 100 cycles.
中文翻译:

氧空位和双电催化剂在锂基正极中激活阴离子氧化还原的协同作用
部分或全部由阴离子-氧化还原反应操作的阴极,例如富锂氧化物 (LRO) 或 Li-O 2电池的空气阴极,由于其高能量密度而具有吸引力。其中,基于锂 (Li 2 O) 的阴极系统作为 LRO 或 Li-O 2的替代品而备受关注电池系统,因为它们在安全、密封的环境中具有高能量密度。然而,由于氧排放导致的循环稳定性差以及充电过程中难以激活阴离子-氧化还原反应,锂正极的实际应用仍然受到阻碍。在此,我们使用促进析氧反应 (OER) 和氧还原反应 (ORR) 并增强催化剂和锂中的氧空位以实现可逆阴离子氧化还原的催化剂。采用的OER和ORR催化剂分别为Co 3 O 4和Ru掺杂的CeO 2。二氧化铈预计通过其卓越的储氧能力调节氧气,因此被用作补充催化剂。通过热处理 Li 2 O 2产生富含氧空位的中间相Li 2 O 2- x和 Ru 掺杂到 CeO 2中,实现了氧空位增强。通过比较研究,我们发现正极中的氧空位显着提高了锂正极的循环稳定性。此外,Co 3 O 4OER 催化剂对于在锂中引发阴离子氧化还原至关重要;然而,该阴极的可逆操作需要 ORR 催化剂。Co 3 O 4 /Ru–CeO 2 /Li 2 O 2− x电极中氧空位、双 OER/ORR 催化剂和具有氧介导能力的互补催化剂的协同组合导致优异的电化学特性和循环稳定性超过 100 个循环的容量限制为 450 mA hg −1 。
更新日期:2022-11-28
中文翻译:

氧空位和双电催化剂在锂基正极中激活阴离子氧化还原的协同作用
部分或全部由阴离子-氧化还原反应操作的阴极,例如富锂氧化物 (LRO) 或 Li-O 2电池的空气阴极,由于其高能量密度而具有吸引力。其中,基于锂 (Li 2 O) 的阴极系统作为 LRO 或 Li-O 2的替代品而备受关注电池系统,因为它们在安全、密封的环境中具有高能量密度。然而,由于氧排放导致的循环稳定性差以及充电过程中难以激活阴离子-氧化还原反应,锂正极的实际应用仍然受到阻碍。在此,我们使用促进析氧反应 (OER) 和氧还原反应 (ORR) 并增强催化剂和锂中的氧空位以实现可逆阴离子氧化还原的催化剂。采用的OER和ORR催化剂分别为Co 3 O 4和Ru掺杂的CeO 2。二氧化铈预计通过其卓越的储氧能力调节氧气,因此被用作补充催化剂。通过热处理 Li 2 O 2产生富含氧空位的中间相Li 2 O 2- x和 Ru 掺杂到 CeO 2中,实现了氧空位增强。通过比较研究,我们发现正极中的氧空位显着提高了锂正极的循环稳定性。此外,Co 3 O 4OER 催化剂对于在锂中引发阴离子氧化还原至关重要;然而,该阴极的可逆操作需要 ORR 催化剂。Co 3 O 4 /Ru–CeO 2 /Li 2 O 2− x电极中氧空位、双 OER/ORR 催化剂和具有氧介导能力的互补催化剂的协同组合导致优异的电化学特性和循环稳定性超过 100 个循环的容量限制为 450 mA hg −1 。



























 京公网安备 11010802027423号
京公网安备 11010802027423号