当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Antimicrobial, cytotoxic, and insulin-releasing activities of the amphibian host-defense peptide ocellatin-3N and its L-lysine-substituted analogs
Journal of Peptide Science ( IF 2.1 ) Pub Date : 2022-11-24 , DOI: 10.1002/psc.3463 J Michael Conlon 1 , Lauren Hunter 1 , Samir Attoub 2 , Bruno Casciaro 3 , Milena Mechkarska 4 , Yasser H A Abdel-Wahab 1
Journal of Peptide Science ( IF 2.1 ) Pub Date : 2022-11-24 , DOI: 10.1002/psc.3463 J Michael Conlon 1 , Lauren Hunter 1 , Samir Attoub 2 , Bruno Casciaro 3 , Milena Mechkarska 4 , Yasser H A Abdel-Wahab 1
Affiliation
|
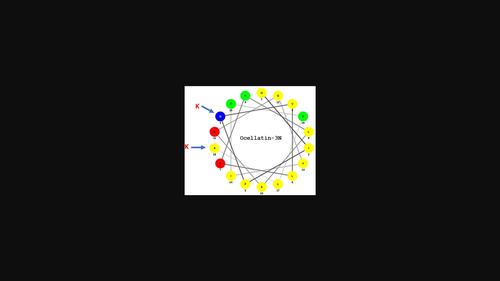
The host-defense peptide ocellatin-3N (GIFDVLKNLAKGVITSLAS.NH2), first isolated from the Caribbean frog Leptodactylus nesiotus, inhibited growth of clinically relevant Gram-positive and Gram-negative bacteria as well as a strain of the major emerging yeast pathogen Candida parapsilosis. Increasing cationicity while maintaining amphipathicity by the substitution Asp4→Lys increased potency against the microorganisms by between 4- and 16-fold (MIC ≤3 μM) compared with the naturally occurring peptide. The substitution Ala18→Lys and the double substitution Asp4→Lys and Ala18→Lys had less effects on potency. The [D4K] analog also showed 2.5- to 4-fold greater cytotoxic potency against non-small-cell lung adenocarcinoma A549 cells, breast adenocarcinoma MDA-MB-231 cells, and colorectal adenocarcinoma HT-29 cells (LC50 values in the range of 12–20 μM) compared with ocellatin-3N but was less hemolytic to mouse erythrocytes. However, the peptide showed no selectivity for tumor-derived cells [LC50 = 20 μM for human umbilical vein endothelial cells (HUVECs)]. Ocellatin-3N and [D4K]ocellatin-3N stimulated the release of insulin from BRIN-BD11 clonal β-cells at concentrations ≥1 nM, and [A18K]ocellatin-3N, at concentrations ≥0.1 nM. No peptide stimulated the release of lactate dehydrogenase at concentrations up to 3 μM, indicating that plasma membrane integrity had been preserved. The three peptides produced an increase in intracellular [Ca2+] in BRIN-BD11 cells when incubated at a concentration of 1 μM. In view of its high insulinotropic potency and relatively low hemolytic activity, the [A18K] ocellatin analog may represent a template for the design of agents with therapeutic potential for the treatment of patients with type 2 diabetes.
中文翻译:

两栖动物宿主防御肽 ocellatin-3N 及其 L-赖氨酸取代类似物的抗菌、细胞毒性和胰岛素释放活性
宿主防御肽 ocellatin-3N (GIFDVLKNLAKGVITSLAS.NH 2 ),首先从加勒比蛙Leptodactylus nesiotus中分离出来,抑制临床相关的革兰氏阳性和革兰氏阴性细菌以及主要新兴酵母病原体近平滑念珠菌菌株的生长. 与天然存在的肽相比,通过替代 Asp 4 →Lys 增加阳离子性同时保持两亲性,对微生物的效力增加了 4 到 16 倍(MIC ≤3 μM)。替代 Ala 18 →Lys 和双重替代 Asp 4 →Lys 和 Ala 18→Lys 对效力的影响较小。[D4K] 类似物还显示出对非小细胞肺腺癌 A549 细胞、乳腺癌 MDA-MB-231 细胞和结直肠腺癌 HT-29 细胞的 2.5 至 4 倍更高的细胞毒性(LC 50 值在范围内12-20 μM)与 ocellatin-3N 相比,但对小鼠红细胞的溶血性较低。然而,该肽对肿瘤衍生细胞没有选择性 [LC 50 = 20 μM 用于人脐静脉内皮细胞 (HUVEC)]。Ocellatin-3N 和 [D4K]ocellatin-3N 在浓度≥1 nM 时刺激 BRIN-BD11 克隆 β 细胞释放胰岛素,在浓度≥0.1 nM 时刺激 [A18K]ocellatin-3N。在高达 3 μM 的浓度下,没有肽会刺激乳酸脱氢酶的释放,这表明质膜完整性得到了保护。当以 1 μM 的浓度孵育时,这三种肽会增加 BRIN-BD11 细胞的细胞内 [Ca 2+ ]。鉴于其高促胰岛素效力和相对低的溶血活性,[A18K] ocellatin 类似物可能代表设计具有治疗 2 型糖尿病患者治疗潜力的药物的模板。
更新日期:2022-11-24
中文翻译:

两栖动物宿主防御肽 ocellatin-3N 及其 L-赖氨酸取代类似物的抗菌、细胞毒性和胰岛素释放活性
宿主防御肽 ocellatin-3N (GIFDVLKNLAKGVITSLAS.NH 2 ),首先从加勒比蛙Leptodactylus nesiotus中分离出来,抑制临床相关的革兰氏阳性和革兰氏阴性细菌以及主要新兴酵母病原体近平滑念珠菌菌株的生长. 与天然存在的肽相比,通过替代 Asp 4 →Lys 增加阳离子性同时保持两亲性,对微生物的效力增加了 4 到 16 倍(MIC ≤3 μM)。替代 Ala 18 →Lys 和双重替代 Asp 4 →Lys 和 Ala 18→Lys 对效力的影响较小。[D4K] 类似物还显示出对非小细胞肺腺癌 A549 细胞、乳腺癌 MDA-MB-231 细胞和结直肠腺癌 HT-29 细胞的 2.5 至 4 倍更高的细胞毒性(LC 50 值在范围内12-20 μM)与 ocellatin-3N 相比,但对小鼠红细胞的溶血性较低。然而,该肽对肿瘤衍生细胞没有选择性 [LC 50 = 20 μM 用于人脐静脉内皮细胞 (HUVEC)]。Ocellatin-3N 和 [D4K]ocellatin-3N 在浓度≥1 nM 时刺激 BRIN-BD11 克隆 β 细胞释放胰岛素,在浓度≥0.1 nM 时刺激 [A18K]ocellatin-3N。在高达 3 μM 的浓度下,没有肽会刺激乳酸脱氢酶的释放,这表明质膜完整性得到了保护。当以 1 μM 的浓度孵育时,这三种肽会增加 BRIN-BD11 细胞的细胞内 [Ca 2+ ]。鉴于其高促胰岛素效力和相对低的溶血活性,[A18K] ocellatin 类似物可能代表设计具有治疗 2 型糖尿病患者治疗潜力的药物的模板。


























 京公网安备 11010802027423号
京公网安备 11010802027423号