European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2022-11-25 , DOI: 10.1016/j.ejmech.2022.114952 Alessandro Papa 1 , Silvia Pasquini 2 , Francesca Galvani 3 , Mariarosaria Cammarota 4 , Chiara Contri 5 , Gabriele Carullo 6 , Sandra Gemma 1 , Anna Ramunno 7 , Stefania Lamponi 1 , Beatrice Gorelli 6 , Simona Saponara 6 , Katia Varani 5 , Marco Mor 8 , Giuseppe Campiani 1 , Francesca Boscia 4 , Fabrizio Vincenzi 5 , Alessio Lodola 3 , Stefania Butini 1
|
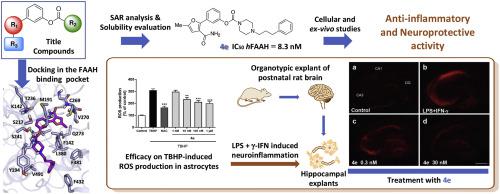
The neuroprotective performance against neuroinflammation of the endocannabinoid system (ECS) can be remarkably improved by indirect stimulation mediated by the pharmacological inhibition of the key ECS catabolic enzyme fatty acid amide hydrolase (FAAH). Based on our previous works and aiming to discover new selective FAAH inhibitors , we herein reported a new series of carbamate-based FAAH inhibitors (4a-t) which showed improved drug disposition properties compared to the previously reported analogues 2a-b. The introduction of ionizable functions allowed us to obtain new FAAH inhibitors of nanomolar potency characterized by good water solubility and chemical stability at physiological pH. Interesting structure-activity relationships (SARs), deeply analyzed by molecular docking and molecular dynamic (MD) simulations, were obtained. All the newly developed inhibitors showed an excellent selectivity profile evaluated against monoacylglycerol lipase and cannabinoid receptors. The reversible mechanism of action was determined by a rapid dilution assay. Absence of toxicity was confirmed in mouse fibroblasts NIH3T3 (for compounds 4e, 4g, 4n-o, and 4s) and in human astrocytes cell line 1321N1 (for compounds 4e, 4n, and 4s). The absence of undesired cardiac effects was also confirmed for compound 4n. Selected analogues (compounds 4e, 4g, 4n, and 4s) were able to reduce oxidative stress in 1321N1 astrocytes and exhibited notable neuroprotective effects when tested in an ex vivo model of neuroinflammation.
中文翻译:

开发具有改善类药物特性的强效选择性 FAAH 抑制剂作为治疗神经炎症的潜在工具
通过由关键 ECS 分解代谢酶脂肪酸酰胺水解酶 (FAAH) 的药理学抑制介导的间接刺激,可以显着改善内源性大麻素系统 (ECS) 对神经炎症的神经保护性能。基于我们之前的工作,旨在发现新的选择性 FAAH 抑制剂,我们在此报道了一系列新的基于氨基甲酸酯的 FAAH 抑制剂 ( 4a - t ),与之前报道的类似物2a - b相比,它显示出改善的药物处置特性。. 电离功能的引入使我们能够获得具有纳摩尔效力的新型 FAAH 抑制剂,其特点是在生理 pH 值下具有良好的水溶性和化学稳定性。获得了通过分子对接和分子动力学 (MD) 模拟深入分析的有趣构效关系 (SAR)。所有新开发的抑制剂都显示出针对单酰基甘油脂肪酶和大麻素受体的出色选择性。可逆作用机制通过快速稀释测定确定。在小鼠成纤维细胞 NIH3T3(对于化合物4e、4g、4n - o和4s)和人星形胶质细胞细胞系 1321N1(对于化合物4e、4n和4s)。化合物4n也证实没有不良的心脏作用。选定的类似物(化合物4e、4g、4n和4s)能够减少 1321N1 星形胶质细胞的氧化应激,并在离体神经炎症模型中测试时表现出显着的神经保护作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号