European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2022-11-24 , DOI: 10.1016/j.ejmech.2022.114939 Ruo-Lan Zhou 1 , Zhiran Ju 1 , Christophe Pannecouque 2 , Erik De Clercq 2 , Shuai Wang 3 , Fen-Er Chen 4
|
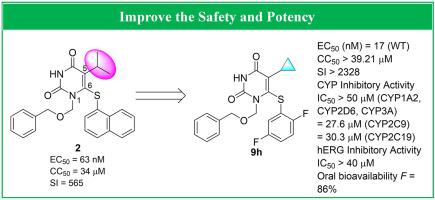
Members of the HEPT class are potential non-nucleoside inhibitors of HIV-1 reverse transcriptase. Our previously disclosed one representative HEPT analog 2 produced potent inhibitory activity against wild-type HIV-1 (EC50 = 63.0 nM), but its high cytotoxicity and low selectivity index still needs to be improved (CC50 = 34.0 μM, SI = 565). In this work, a series of novel cyclopropyl-substituted HEPT analogs were developed by substituting a cyclopropyl ring for the isopropyl group at the C-5 position of 2 with the purpose of improving its potency and safety. Of this series, the most potent compound 9h featuring a 2,5-fluoro substitution on the C-6 benzene ring exerted significantly increased inhibitory activity toward wild-type HIV-1 (EC50 = 0.017 μM), which was 4-fold more active than the lead compound 2. The cytotoxicity of 9h was also reduced with much higher selectivity index (SI > 2328). This compound possessed good pharmacokinetics profiles and potential safety: (1) No obvious in vitro inhibition effect toward CYP enzyme and hERG was observed in 9h; (2) The single-dose acute toxicity test did not induce mice death and obvious pathological damage; (3) Excellent oral bioavailability of 9h (F = 86%) in rats was unveiled. These results provide valuable guidance for further development of HEPT anti-HIV-1 drugs.
中文翻译:

具有增强效力和安全性的新型 HEPT 类似物的结构导向设计:从异丙基-HEPT 到环丙基-HEPT
HEPT 类的成员是 HIV-1 逆转录酶的潜在非核苷抑制剂。我们先前公开的一种代表性HEPT类似物2对野生型HIV-1产生了有效的抑制活性(EC 50 = 63.0 nM),但其高细胞毒性和低选择性指数仍有待提高(CC 50 = 34.0 μM,SI = 565 ). 在这项工作中,通过在2的 C-5 位置用环丙基环取代异丙基,开发了一系列新型环丙基取代的 HEPT 类似物,目的是提高其效力和安全性。在这个系列中,最有效的化合物9h以 C-6 苯环上的 2,5-氟取代为特征,对野生型 HIV-1 (EC 50 = 0.017 μM) 的抑制活性显着增加,其活性是先导化合物2 的 4 倍。9h的细胞毒性也随着更高的选择性指数 (SI > 2328) 而降低。该化合物具有良好的药代动力学特征和潜在的安全性:(1)体外9h对CYP酶和hERG无明显抑制作用;(2)单剂量急性毒性试验未引起小鼠死亡和明显病理损伤;(3) 优秀的口服生物利用度为9h ( F =86%) 在老鼠身上揭开了面纱。这些结果为进一步开发HEPT抗HIV-1药物提供了有价值的指导。


























 京公网安备 11010802027423号
京公网安备 11010802027423号