当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Probing B–X to B–H conversions and applications in C–F bond activation catalysis
Dalton Transactions ( IF 4 ) Pub Date : 2022-11-24 , DOI: 10.1039/d2dt03588j Amir Yeganeh-Salman 1 , Iris Elser 1 , Karlee L Bamford 1 , Daniel Ebanks 1 , Douglas W Stephan 1
Dalton Transactions ( IF 4 ) Pub Date : 2022-11-24 , DOI: 10.1039/d2dt03588j Amir Yeganeh-Salman 1 , Iris Elser 1 , Karlee L Bamford 1 , Daniel Ebanks 1 , Douglas W Stephan 1
Affiliation
|
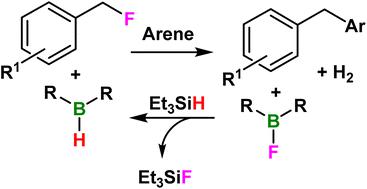
Herein we exploit a catalytic amount of [Ph3C]+ to initiate B–X to B–H bond conversion with Et3SiH. This was applied to 6 haloboranes. However, 9-X-9-borabicyclo[3.3.1]nonane (B-X-9-BBN, X = F, Br) reacts directly with silane. Thus, C–F bond activation of benzyl fluorides in the presence of arenes afforded the Friedel–Crafts (FC) products using B–H-9-BBN in the presence of Et3SiH. This catalysis was probed with a range of arenes and several benzyl fluoride derivatives. The protocol is simple, cheap and a convenient route to 1,1-diarylmethanes from benzyl fluorides in good to excellent yields (up to 99%) under mild conditions.
中文翻译:

B-X 到 B-H 转化的探索及其在 C-F 键活化催化中的应用
在此,我们利用催化量的 [Ph 3 C] +启动 B-X 到 B-H 键与 Et 3 SiH 的转化。这适用于 6 种卤代硼烷。然而,9-X-9-borabicyclo[3.3.1]nonane (BX-9-BBN, X = F, Br) 直接与硅烷反应。因此,在存在芳烃的情况下,苄基氟化物的 C-F 键活化在 Et 3 SiH 存在的情况下使用 B-H-9-BBN 提供 Friedel-Crafts (FC) 产品。用一系列芳烃和几种苄基氟衍生物探索了这种催化作用。该方案简单、便宜且是从苄基氟化物到 1,1-二芳基甲烷的便捷途径,在温和条件下产量高(高达 99%)。
更新日期:2022-11-29
中文翻译:

B-X 到 B-H 转化的探索及其在 C-F 键活化催化中的应用
在此,我们利用催化量的 [Ph 3 C] +启动 B-X 到 B-H 键与 Et 3 SiH 的转化。这适用于 6 种卤代硼烷。然而,9-X-9-borabicyclo[3.3.1]nonane (BX-9-BBN, X = F, Br) 直接与硅烷反应。因此,在存在芳烃的情况下,苄基氟化物的 C-F 键活化在 Et 3 SiH 存在的情况下使用 B-H-9-BBN 提供 Friedel-Crafts (FC) 产品。用一系列芳烃和几种苄基氟衍生物探索了这种催化作用。该方案简单、便宜且是从苄基氟化物到 1,1-二芳基甲烷的便捷途径,在温和条件下产量高(高达 99%)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号