当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synergistic Effects of Keggin-Type Phosphotungstic Acid-Supported Single-Atom Catalysts in a Fast NH3-SCR Reaction
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2022-11-22 , DOI: 10.1021/acs.inorgchem.2c02759 Chun-Hong Lin 1, 2, 3 , Rui-Cheng Qin 1 , Ning Cao 3 , Dan Wang 1 , Chun-Guang Liu 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2022-11-22 , DOI: 10.1021/acs.inorgchem.2c02759 Chun-Hong Lin 1, 2, 3 , Rui-Cheng Qin 1 , Ning Cao 3 , Dan Wang 1 , Chun-Guang Liu 1
Affiliation
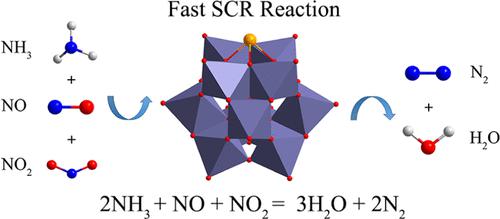
|
Fast selective catalytic reduction of nitrogen oxide with ammonia (NH3-SCR) (2NH3 + NO2 + NO → 2N2 + 3H2O) has aroused great interest in recent years because it is inherently faster than the standard NH3-SCR reaction (4NO + 4NH3 + O2 → 4N2 + 6H2O). In the present paper, the mechanism of the fast NH3-SCR reaction catalyzed by a series of single-atom catalysts (SACs), M1/PTA SACs (PTA = Keggin-type phosphotungstic acid, M = Mn, Fe, Co, Ni, Ru, Rh, Pd, Ir, and Pt), has been systematically studied by means of density functional theory (DFT) calculations. Molecular geometry and electronic structural analysis show that Jahn–Teller distortion effects promote an electron transfer process from N–H bonding orbitals of the NH3 molecule to the symmetry-allowed d orbitals (dxy and dx2–y2) of the single metal atom, which effectively weakens the N–H bond of the adsorbed NH3 molecule. The calculated free energy profiles along the favorable catalytic path show that decomposition of NH3 to *NH2 and *H species and decomposition of *NHNOH into N2 and H2O have high free energy barriers in the whole fast NH3-SCR path. A good synergistic effect between the Brønsted acid site (surface oxygen atom in the PTA support) and the Lewis acid site (single metal atom) effectively enhances the decomposition of NH3 to *NH2 and *H species. M1/PTA SACs (M = Ru, Rh, Pd, and Pt) were found to have potential for fast NH3-SCR reaction because of the relatively small free energy barrier and strong thermodynamic driving forces. We hope our computational results could provide some new ideas for designing and fabricating fast NH3-SCR catalysts with high activity.
中文翻译:

Keggin 型磷钨酸负载单原子催化剂在快速 NH3-SCR 反应中的协同效应
用氨快速选择性催化还原氮氧化物(NH 3 -SCR)(2NH 3 + NO 2 + NO → 2N 2 + 3H 2 O)近年来引起了极大的兴趣,因为它本质上比标准的NH 3 -SCR更快反应(4NO + 4NH 3 + O 2 → 4N 2 + 6H 2 O)。在本文中,一系列单原子催化剂(SACs)催化的快速NH 3 -SCR反应的机理,M 1/PTA SACs(PTA = Keggin 型磷钨酸,M = Mn、Fe、Co、Ni、Ru、Rh、Pd、Ir 和 Pt)已通过密度泛函理论 (DFT) 计算进行了系统研究。分子几何学和电子结构分析表明,Jahn-Teller 畸变效应促进了电子从 NH 3分子的 N-H 键轨道到单个分子的对称允许 d 轨道(d xy和 d x 2 –y 2)的电子转移过程金属原子,它有效地削弱了吸附的 NH 3分子的 N-H 键。沿有利催化路径计算的自由能分布表明 NH 3分解为 *NH 2和*H物种以及*NHNOH分解成N 2和H 2 O在整个快速NH 3 -SCR路径中具有高自由能垒。Brønsted酸位(PTA载体中的表面氧原子)和Lewis酸位(单个金属原子)之间良好的协同作用有效地促进了NH 3分解为*NH 2和*H物种。M 1 /PTA SAC(M = Ru、Rh、Pd 和 Pt)被发现具有快速 NH 3 -SCR 反应的潜力,因为它具有相对较小的自由能垒和较强的热力学驱动力。我们希望我们的计算结果能够为设计和制造快速NH 3提供一些新的思路。-高活性SCR催化剂。
更新日期:2022-11-22
中文翻译:

Keggin 型磷钨酸负载单原子催化剂在快速 NH3-SCR 反应中的协同效应
用氨快速选择性催化还原氮氧化物(NH 3 -SCR)(2NH 3 + NO 2 + NO → 2N 2 + 3H 2 O)近年来引起了极大的兴趣,因为它本质上比标准的NH 3 -SCR更快反应(4NO + 4NH 3 + O 2 → 4N 2 + 6H 2 O)。在本文中,一系列单原子催化剂(SACs)催化的快速NH 3 -SCR反应的机理,M 1/PTA SACs(PTA = Keggin 型磷钨酸,M = Mn、Fe、Co、Ni、Ru、Rh、Pd、Ir 和 Pt)已通过密度泛函理论 (DFT) 计算进行了系统研究。分子几何学和电子结构分析表明,Jahn-Teller 畸变效应促进了电子从 NH 3分子的 N-H 键轨道到单个分子的对称允许 d 轨道(d xy和 d x 2 –y 2)的电子转移过程金属原子,它有效地削弱了吸附的 NH 3分子的 N-H 键。沿有利催化路径计算的自由能分布表明 NH 3分解为 *NH 2和*H物种以及*NHNOH分解成N 2和H 2 O在整个快速NH 3 -SCR路径中具有高自由能垒。Brønsted酸位(PTA载体中的表面氧原子)和Lewis酸位(单个金属原子)之间良好的协同作用有效地促进了NH 3分解为*NH 2和*H物种。M 1 /PTA SAC(M = Ru、Rh、Pd 和 Pt)被发现具有快速 NH 3 -SCR 反应的潜力,因为它具有相对较小的自由能垒和较强的热力学驱动力。我们希望我们的计算结果能够为设计和制造快速NH 3提供一些新的思路。-高活性SCR催化剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号