当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Carboxylic Acids as Adaptive Functional Groups in Metallaphotoredox Catalysis
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2022-11-22 , DOI: 10.1021/acs.accounts.2c00607 Sebastian B Beil 1 , Tiffany Q Chen 1 , Nicholas E Intermaggio 1 , David W C MacMillan 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2022-11-22 , DOI: 10.1021/acs.accounts.2c00607 Sebastian B Beil 1 , Tiffany Q Chen 1 , Nicholas E Intermaggio 1 , David W C MacMillan 1
Affiliation
|
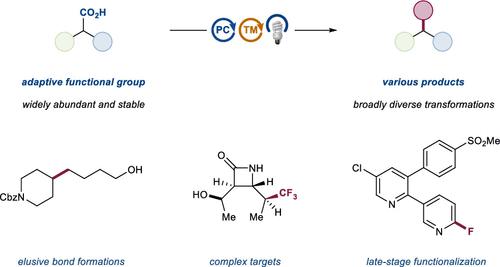
The development of palladium-catalyzed cross-coupling methods for the activation of C(sp2)–Br bonds facilitated access to arene-rich molecules, enabling a concomitant increase in the prevalence of this structural motif in drug molecules in recent decades. Today, there is a growing appreciation of the value of incorporating saturated C(sp3)-rich scaffolds into pharmaceutically active molecules as a means to achieve improved solubility and physiological stability, providing the impetus to develop new coupling strategies to access these challenging motifs in the most straightforward way possible. As an alternative to classical two-electron chemistry, redox chemistry can enable access to elusive transformations, most recently, by interfacing abundant first-row transition-metal catalysis with photoredox catalysis. As such, the functionalization of ubiquitous and versatile functional handles such as (aliphatic) carboxylic acids via metallaphotoredox catalysis has emerged as a valuable field of research over the past eight years.
中文翻译:

羧酸作为金属光氧化还原催化中的自适应官能团
用于激活 C(sp 2 )-Br 键的钯催化交叉偶联方法的发展促进了富含芳烃的分子的获得,使得近几十年来药物分子中这种结构基序的普遍性随之增加。如今,人们越来越认识到将富含 C(sp 3 ) 的饱和支架纳入药物活性分子中作为提高溶解度和生理稳定性的一种手段的价值,这为开发新的偶联策略以获取这些具有挑战性的基序提供了动力。最直接的方式。作为经典双电子化学的替代品,氧化还原化学最近通过将丰富的第一行过渡金属催化与光氧化还原催化相结合,可以实现难以捉摸的转变。因此,在过去八年中,通过金属光氧化还原催化对(脂肪族)羧酸等普遍存在的多功能功能手柄进行功能化已成为一个有价值的研究领域。
更新日期:2022-11-22
中文翻译:

羧酸作为金属光氧化还原催化中的自适应官能团
用于激活 C(sp 2 )-Br 键的钯催化交叉偶联方法的发展促进了富含芳烃的分子的获得,使得近几十年来药物分子中这种结构基序的普遍性随之增加。如今,人们越来越认识到将富含 C(sp 3 ) 的饱和支架纳入药物活性分子中作为提高溶解度和生理稳定性的一种手段的价值,这为开发新的偶联策略以获取这些具有挑战性的基序提供了动力。最直接的方式。作为经典双电子化学的替代品,氧化还原化学最近通过将丰富的第一行过渡金属催化与光氧化还原催化相结合,可以实现难以捉摸的转变。因此,在过去八年中,通过金属光氧化还原催化对(脂肪族)羧酸等普遍存在的多功能功能手柄进行功能化已成为一个有价值的研究领域。



























 京公网安备 11010802027423号
京公网安备 11010802027423号