Journal of Catalysis ( IF 7.3 ) Pub Date : 2022-11-21 , DOI: 10.1016/j.jcat.2022.11.023 Reece Paterson , Husam Y. Alharbi , Corinne Wills , Thomas W. Chamberlain , Richard A Bourne , Anthony Griffiths , Sean M. Collins , Kejun Wu , Matthew D. Simmons , Robert Menzel , Alexander F. Masey , Julian G. Knight , Simon Doherty
|
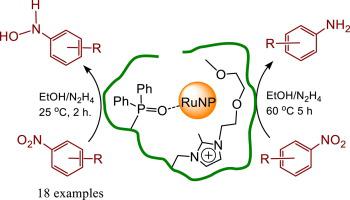
RuNPs stabilised by a polymer immobilised ionic liquid derived from co-polymerisation of a PEG-substituted imidazolium-based styrene monomer and diphenyl(4-vinylphenyl)phosphine oxide, RuNP@O = PPh2-PEGPIILS, (2) is a remarkably efficient and selective catalyst for the hydrazine hydrate-mediated partial reduction of nitroarenes to the corresponding N-arylhydoxylamine. Near quantitative conversion to N-phenylhydroxylamine with > 99 % selectivity was obtained after only 2 h when the reaction was conducted at 25 °C in ethanol under an inert atmosphere using 0.1 mol% catalyst. Under these conditions, the composition-time profile showed that the reduction occurred via the direct pathway whereas reactions in air gave a mixture of azoxy-based products due to competing condensation resulting from reversible formation of N-phenylhydroxylamine. The initial TOF of 6,100 h−1 obtained after 10 min at 40 °C with 0.1 mol% 2 is among the highest to be reported for the metal nanoparticle catalysed reduction of nitrobenzene to N-phenylhydroxylamine and a significant improvement on 5 wt% Ru/C which gave a modest conversion of 21 % (initial TOF = 240 h−1) to a mixture of N-phenylhydroxylamine and aniline. A broad range of substituted N-aryl and N-heteroaryl nitroarenes were reduced to the corresponding N-arylhydroxylamine in high yield and with excellent selectivity by adjusting the reaction times. However, reduction of electron rich amino-substituted nitroarenes was extremely slow and resulted in reduction to the aniline with no evidence for the corresponding hydroxylamine. Complete reduction of amino substituted nitroarene is proposed to be facilitated by amine-assisted elimination of hydroxide from the hydroxylamine to afford a readily reducible quinondiimine-derived iminium intermediate that reacts with a surface hydride to liberate the amine. Under optimum conditions the catalyst could be reused five times for the reduction of nitrobenzene to N-phenylhydroxylamine with no detectable change in activity and only slight decrease in selectivity.
中文翻译:

氧化膦修饰的聚合物固定化离子液体稳定的钌纳米颗粒催化硝基芳烃高效选择性部分还原为 N-芳基羟胺
由 PEG 取代的咪唑基苯乙烯单体和二苯基(4-乙烯基苯基)氧化膦的共聚合衍生的聚合物固定化离子液体稳定的 RuNPs,RuNP@O = PPh 2 -PEGPIILS,( 2 ) 是一种非常有效和用于水合肼介导的硝基芳烃部分还原为相应的N-芳基羟胺的选择性催化剂。接近定量转化为N当反应在惰性气氛下于 25 °C 在乙醇中使用 0.1 mol% 催化剂进行时,仅 2 小时后就获得了具有 > 99% 选择性的-苯基羟胺。在这些条件下,组成-时间曲线表明还原是通过直接途径发生的,而空气中的反应由于N-苯基羟胺的可逆形成导致的竞争缩合而产生基于氧化氮基的产物的混合物。在 40 °C 下 10 分钟后,0.1 mol% 2的初始 TOF 为 6,100 h −1,是已报道的金属纳米颗粒催化硝基苯还原为N的最高时间之一-苯基羟胺和 5 wt% Ru/C 的显着改进,其将 21%(初始 TOF = 240 h -1 )适度转化为N -苯基羟胺和苯胺的混合物。广泛的取代N -芳基和N -杂芳基硝基芳烃被还原为相应的N-芳基羟胺高产率和通过调整反应时间具有优异的选择性。然而,富电子氨基取代的硝基芳烃的还原非常缓慢,导致还原为苯胺,没有相应羟胺的证据。氨基取代的硝基芳烃的完全还原被认为是通过胺辅助从羟胺中消除氢氧化物来促进的,以提供易于还原的醌二亚胺衍生的亚胺中间体,该中间体与表面氢化物反应以释放胺。在最佳条件下,催化剂可重复使用五次,将硝基苯还原为N-苯基羟胺,活性没有明显变化,选择性仅略有下降。


























 京公网安备 11010802027423号
京公网安备 11010802027423号