当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ring-expansion from tellurophenes to telluropyrans: inhibition of C–Te bond cleavages in transition metal-catalyzed reactions
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-11-22 , DOI: 10.1039/d2qo01732f Si Liu 1 , Zhanglang Zhou 1 , Jing Fang 1 , Min Wang 1 , Hao Zong 1 , Weinan Chen 1 , Gang Zhou 1
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-11-22 , DOI: 10.1039/d2qo01732f Si Liu 1 , Zhanglang Zhou 1 , Jing Fang 1 , Min Wang 1 , Hao Zong 1 , Weinan Chen 1 , Gang Zhou 1
Affiliation
|
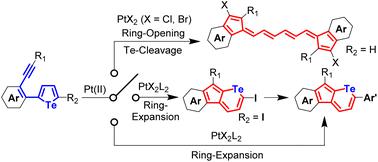
Tellurium-fused aromatic hydrocarbons have attracted extensive attention due to their unique properties as well as the synthetic challenges. However, the synthesis of organotellurium molecules falls much behind those of other chalcogen-containing compounds since the synthetic methods to build C–S and C–Se bonds always fail to extend the C–Te bonds. Herein, a PtCl2-catalyzed ring-expansion reaction is attempted on tellurophene derivatives. However, tellurium-free vinylogous pentafulvalene analogues instead of telluropyrans are produced unexpectedly. To prevent the Pt–Te interactions and avoid the C–Te bond cleavage, bulky phosphine ligands are incorporated into the PtCl2 catalyst. A ring-expansion cyclization towards neutral telluropyran derivatives from the corresponding tellurophene compounds has been thus developed. The mechanisms of both the ring-opening and the ring-expansion reactions are proposed and the pathways are verified by density functional theory calculations. Furthermore, the tolerance of the obtained telluropyran derivatives in metal-related reagents is further investigated and the conjugation can be facilely extended via various transition metal-catalyzed coupling reactions. Therefore, our work provides not only a synthetic methodology towards telluropyran-fused polycyclic aromatic hydrocarbons, but also an effective pathway to avoid the C–Te bond cleavages in transition metal-catalyzed coupling reactions.
中文翻译:

从碲吩到碲吡喃的环扩展:过渡金属催化反应中 C-Te 键断裂的抑制
碲融合芳烃由于其独特的性质和合成挑战而受到广泛关注。然而,有机碲分子的合成远远落后于其他含硫族元素的化合物,因为构建 C-S 和 C-Se 键的合成方法总是无法延长 C-Te 键。在此,尝试对碲吩衍生物进行PtCl 2催化的扩环反应。然而,出乎意料地产生了不含碲的插烯五富瓦烯类似物而不是碲吡喃。为了防止 Pt-Te 相互作用并避免 C-Te 键断裂,将大体积膦配体掺入 PtCl 2催化剂。因此开发了从相应的碲吩化合物向中性碲吡喃衍生物的扩环环化。提出了开环和扩环反应的机理,并通过密度泛函理论计算验证了途径。此外,进一步研究了所得碲吡喃衍生物在金属相关试剂中的耐受性,并且可以通过各种过渡金属催化的偶联反应轻松扩展缀合。因此,我们的工作不仅提供了一种合成碲吡喃稠合多环芳烃的方法,而且提供了避免过渡金属催化偶联反应中 C-Te 键断裂的有效途径。
更新日期:2022-11-22
中文翻译:

从碲吩到碲吡喃的环扩展:过渡金属催化反应中 C-Te 键断裂的抑制
碲融合芳烃由于其独特的性质和合成挑战而受到广泛关注。然而,有机碲分子的合成远远落后于其他含硫族元素的化合物,因为构建 C-S 和 C-Se 键的合成方法总是无法延长 C-Te 键。在此,尝试对碲吩衍生物进行PtCl 2催化的扩环反应。然而,出乎意料地产生了不含碲的插烯五富瓦烯类似物而不是碲吡喃。为了防止 Pt-Te 相互作用并避免 C-Te 键断裂,将大体积膦配体掺入 PtCl 2催化剂。因此开发了从相应的碲吩化合物向中性碲吡喃衍生物的扩环环化。提出了开环和扩环反应的机理,并通过密度泛函理论计算验证了途径。此外,进一步研究了所得碲吡喃衍生物在金属相关试剂中的耐受性,并且可以通过各种过渡金属催化的偶联反应轻松扩展缀合。因此,我们的工作不仅提供了一种合成碲吡喃稠合多环芳烃的方法,而且提供了避免过渡金属催化偶联反应中 C-Te 键断裂的有效途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号