Environment International ( IF 11.8 ) Pub Date : 2022-11-19 , DOI: 10.1016/j.envint.2022.107636 Jiayu Xu 1 , Qiaojian Zhang 1 , Zekang Su 1 , Yu Liu 1 , Tenglong Yan 1 , Yali Zhang 1 , Tiancheng Wang 2 , Xuetao Wei 3 , Zhangjian Chen 1 , Guiping Hu 4 , Tian Chen 5 , Guang Jia 1
|
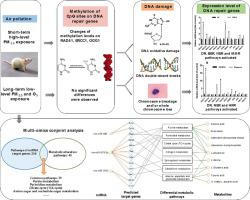
Ambient air pollution was classified as carcinogenic to humans (Group 1) for lung cancer. DNA damage was an important first step in the process of carcinogenesis, and could also be induced by air pollution. In this study, intratracheal instillation and real-time air exposure system were combined to establish SHP (short-term high-level PM2.5) and LLPO (long-term low-level PM2.5 and O3) exposure patterns, respectively. Hierarchical levels of genetic biomarkers were analyzed to explore DNA damage effects in rats. Representative DNA repair genes from different repair pathways were selected to explore the relative expression levels. The methylation level of differentially expressed repair genes were also determined. Besides, miRNA sequencing and non-targeted metabolomic analysis were performed in rat lungs. KEGG and multi-omics analysis were used to explore the potential mechanism of genetic damage under different air pollution patterns. We found that LLPO exposure induced DSBs and chromosome damage. SHP exposure could induce DSBs and DNA oxidative damage, and the effects of genetic damage under this pollution pattern could be repaired by natural repair. Repair genes involved in two pattern were different. SHP exposure could induce higher methylation levels of RAD51, which might be a potential epigenetic mechanism for high-level PM2.5 induced down-regulated expression of RAD51 and DSBs. Besides, 29 overlapped alterations in metabolic pathways were identified by metabolomic and miRNA sequencing, including purine metabolism and pyrimidine metabolism after LLPO exposure. Differential miRNAs expression in lung tissue were associated with apoptosis, DNA damage and damage repair. We concluded that under different air pollution patterns, DNA damage biomarkers and activated targets of DNA damage repair network were both different. The genetic damage effects caused by high-level short-term PM2.5 can be alleviated by natural repair. We provided possible mechanisms by multi-omics which could explain the increased carcinogenic risk caused by air pollution.
中文翻译:

多组学探索不同大气污染模式下的遗传损伤及潜在机制
环境空气污染被归类为人类致癌物(第 1 组)肺癌。DNA 损伤是致癌过程中重要的第一步,也可能由空气污染引起。在这项研究中,气管内滴注和实时空气暴露系统相结合,建立了 SHP(短期高水平 PM 2.5)和 LLPO(长期低水平 PM 2.5和 O 3)曝光模式,分别。分析了遗传生物标志物的层次水平,以探索大鼠的 DNA 损伤效应。选择来自不同修复途径的代表性 DNA 修复基因来探索相对表达水平。还确定了差异表达的修复基因的甲基化水平。此外,在大鼠肺中进行了 miRNA 测序和非靶向代谢组学分析。利用KEGG和多组学分析探索不同空气污染模式下基因损伤的潜在机制。我们发现 LLPO 暴露会导致 DSB 和染色体损伤。小水电暴露可诱导 DSBs 和 DNA 氧化损伤,这种污染模式下的遗传损伤效应可以通过自然修复来修复。两种模式涉及的修复基因不同。2.5诱导RAD51和DSBs表达下调。此外,通过代谢组学和 miRNA 测序鉴定了 29 个代谢途径的重叠改变,包括 LLPO 暴露后的嘌呤代谢和嘧啶代谢。肺组织中差异 miRNA 表达与细胞凋亡、DNA 损伤和损伤修复有关。我们得出结论,在不同的空气污染模式下,DNA 损伤生物标志物和 DNA 损伤修复网络的激活靶点都不同。PM 2.5短期高水平造成的基因损伤效应可通过自然修复得到缓解。我们通过多组学提供了可能的机制,可以解释空气污染导致的致癌风险增加。



























 京公网安备 11010802027423号
京公网安备 11010802027423号