当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydride-Abstraction-Initiated Catalytic Stereoselective Intermolecular Bond-Forming Processes
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2022-11-17 , DOI: 10.1021/acs.accounts.2c00638 Lei Liu 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2022-11-17 , DOI: 10.1021/acs.accounts.2c00638 Lei Liu 1
Affiliation
|
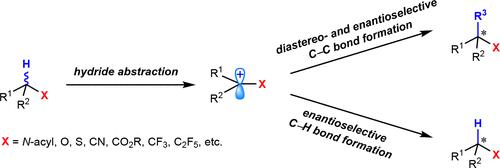
The stereoselective intermolecular bond-forming reactions through the direct manipulation of ubiquitous yet inert C(sp3)–H bonds represent an important and long-standing goal in chemistry. In particular, developing such a stereoselective bimolecular transformation involving carbocation intermediates generated via site-selective hydride abstraction or formal hydride abstraction by organic oxidants would avoid the preinstallation of directing groups and is therefore attractive. Hydride-abstraction-initiated bimolecular transformations have received considerable attention, but existing examples lack stereoselective studies. Prevalent stereoselective studies typically suffer from the narrow substrate scope of specific and highly reactive N-aryl amines and diarylmethanes together with limited synthetic utility. This Account describes our recent advances in the development and synthetic application of hydride-abstraction-initiated stereoselective intermolecular C–C and C–H bond-forming processes with significantly expanded scopes involving structurally diverse N-acyl amines and ethers together with nitriles, esters, and perfluoroalkyl moieties.
中文翻译:

氢化物夺取引发的催化立体选择性分子间键形成过程
通过直接操纵普遍存在但惰性的 C(sp 3 )–H 键进行立体选择性分子间成键反应代表了化学中一个重要且长期存在的目标。特别是,开发这种涉及通过位点选择性氢化物提取或有机氧化剂形式氢化物提取产生的碳阳离子中间体的立体选择性双分子转化将避免定向基团的预安装,因此具有吸引力。氢化物抽象引发的双分子转化受到了相当大的关注,但现有的例子缺乏立体选择性研究。普遍的立体选择性研究通常受到特异性和高反应性N的窄底物范围的影响-芳基胺和二芳基甲烷,合成效用有限。这个帐户描述了我们在氢化物抽象引发的立体选择性分子间 C-C 和 C-H 键形成过程的开发和合成应用方面的最新进展,其范围显着扩大,涉及结构多样的N-酰基胺和醚以及腈、酯、和全氟烷基部分。
更新日期:2022-11-17
中文翻译:

氢化物夺取引发的催化立体选择性分子间键形成过程
通过直接操纵普遍存在但惰性的 C(sp 3 )–H 键进行立体选择性分子间成键反应代表了化学中一个重要且长期存在的目标。特别是,开发这种涉及通过位点选择性氢化物提取或有机氧化剂形式氢化物提取产生的碳阳离子中间体的立体选择性双分子转化将避免定向基团的预安装,因此具有吸引力。氢化物抽象引发的双分子转化受到了相当大的关注,但现有的例子缺乏立体选择性研究。普遍的立体选择性研究通常受到特异性和高反应性N的窄底物范围的影响-芳基胺和二芳基甲烷,合成效用有限。这个帐户描述了我们在氢化物抽象引发的立体选择性分子间 C-C 和 C-H 键形成过程的开发和合成应用方面的最新进展,其范围显着扩大,涉及结构多样的N-酰基胺和醚以及腈、酯、和全氟烷基部分。


























 京公网安备 11010802027423号
京公网安备 11010802027423号