Analytica Chimica Acta ( IF 6.2 ) Pub Date : 2022-11-16 , DOI: 10.1016/j.aca.2022.340634 Yanke Shan 1 , Bin Wang 1 , Huachuan Huang 2 , Keding Yan 3 , Wenzhi Li 4 , Shouyu Wang 5 , Fei Liu 1
|
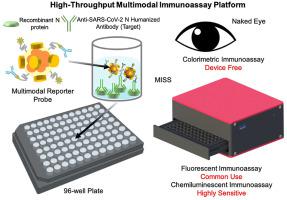
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as a causal agent of Coronavirus Disease 2019 (COVID-19) has led to the global pandemic. Though the real-time reverse transcription polymerase chain reaction (RT-PCR) acting as a gold-standard method has been widely used for COVID-19 diagnostics, it can hardly support rapid on-site applications or monitor the stage of disease development as well as to identify the infection and immune status of rehabilitation patients. To suit rapid on-site COVID-19 diagnostics under various application scenarios with an all-in-one device and simple detection reagents, we propose a high-throughput multimodal immunoassay platform with fluorescent, colorimetric, and chemiluminescent immunoassays on the same portable device and a multimodal reporter probe using quantum dot (QD) microspheres modified with horseradish peroxidase (HRP) coupled with goat anti-human IgG. The recombinant nucleocapsid protein fixed on a 96-well plate works as the capture probe. In the condition with the target under detection, both reporter and capture probes can be bound by such target. When illuminated by excitation light, fluorescence signals from QD microspheres can be collected for target quantification often at a fast speed. Additionally, when pursuing simple detection without using any sensing devices, HRP-catalyzed TMB colorimetric immunoassay is employed; and when pursuing highly sensitive detection, HRP-catalyzed luminol chemiluminescent immunoassay is established. Verified by the anti-SARS-CoV-2 N humanized antibody, the sensitivities of colorimetric, fluorescent, and chemiluminescent immunoassays are respectively 20, 80, and 640 times more sensitive than that of the lateral flow colloidal gold immunoassay strip. Additionally, such a platform can simultaneously detect multiple samples at the same time thus supporting high-throughput sensing; and all these detecting operations can be implemented on-site within 50 min relying on field-operable processing and field-portable devices. Such a high-throughput multimodal immunoassay platform can provide a new all-in-one solution for rapid on-site diagnostics of COVID-19 for different detecting purposes.
中文翻译:

用于快速现场 COVID-19 诊断的便携式高通量多模式免疫分析平台
严重急性呼吸系统综合症冠状病毒 2 (SARS-CoV-2) 作为 2019 年冠状病毒病 (COVID-19) 的病原体已导致全球大流行。尽管作为金标准方法的实时逆转录聚合酶链反应 (RT-PCR) 已广泛用于 COVID-19 诊断,但它很难支持快速现场应用或监测疾病发展阶段以确定康复患者的感染和免疫状态。为了适应各种应用场景下的快速现场 COVID-19 诊断,使用一体机和简单的检测试剂,我们提出了一种高通量多模式免疫分析平台,具有荧光、比色、在同一个便携式设备上进行化学发光免疫测定,以及使用辣根过氧化物酶 (HRP) 和山羊抗人 IgG 修饰的量子点 (QD) 微球的多模式报告探针。固定在 96 孔板上的重组核衣壳蛋白作为捕获探针。在检测目标的条件下,报告探针和捕获探针都可以被该目标结合。当被激发光照射时,通常可以快速收集来自 QD 微球的荧光信号以进行目标量化。此外,在追求不使用任何传感设备的简单检测时,采用HRP催化的TMB比色免疫分析;在追求高灵敏度检测的同时,建立了HRP催化的鲁米诺化学发光免疫分析法。经抗SARS-CoV-2 N人源化抗体验证,比色法、荧光法和化学发光法的灵敏度分别是侧流胶体金免疫试纸条的20、80和640倍。此外,这样的平台可以同时检测多个样本,从而支持高通量传感;所有这些检测操作都可以在50分钟内在现场完成,依靠现场可操作的处理和现场便携式设备。这种高通量多模式免疫分析平台可以为不同检测目的的 COVID-19 快速现场诊断提供一种新的一体化解决方案。这样的平台可以同时检测多个样本,从而支持高通量传感;所有这些检测操作都可以在50分钟内在现场完成,依靠现场可操作的处理和现场便携式设备。这种高通量多模式免疫分析平台可以为不同检测目的的 COVID-19 快速现场诊断提供一种新的一体化解决方案。这样的平台可以同时检测多个样本,从而支持高通量传感;所有这些检测操作都可以在50分钟内在现场完成,依靠现场可操作的处理和现场便携式设备。这种高通量多模式免疫分析平台可以为不同检测目的的 COVID-19 快速现场诊断提供一种新的一体化解决方案。



























 京公网安备 11010802027423号
京公网安备 11010802027423号