当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adjusting the balance between hydrogen and chalcogen bonds
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2022-11-16 , DOI: 10.1039/d2cp04591e Steve Scheiner 1
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2022-11-16 , DOI: 10.1039/d2cp04591e Steve Scheiner 1
Affiliation
|
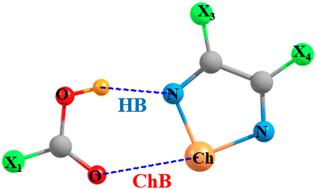
A complex is assembled which pairs a carboxyl group of X1COOH with a 1,2,5-chalcogenadiazole ring containing substituents on its C atoms. The OH of the carboxyl group donates a proton to a N atom of the ring to form a OH⋯N H-bond (HB), while its carbonyl O engages in a Y⋯O chalcogen bond (ChB) with the ring in which Y = S, Se, Te. The ChB is strengthened by enlarging the size of the Y atom from S to Se to Te. Placement of an electron-withdrawing group (EWG) X1 on the acid strengthens the HB while weakening the ChB; the reverse occurs when EWGs are placed on the ring. By selection of the proper substituents on the two units, it is possible to achieve a near perfect balance between the strengths of these two bonds. These bond strengths are also reflected in the NMR spectroscopic properties of the chemical shielding of the various atoms and the coupling between the nuclei directly involved in each bond.
中文翻译:

调整氢键和硫属键之间的平衡
组装了一个络合物,它将 X 1 COOH 的羧基与其 C 原子上含有取代基的 1,2,5-硫属二唑环配对。羧基的 OH 向环的 N 原子提供一个质子,形成 OH⋯N 氢键 (HB),而其羰基 O 与 Y⋯O 硫属元素键 (ChB) 结合,其中 Y = S、Se、Te。通过将 Y 原子的尺寸从 S 扩大到 Se 再到 Te,ChB 得到加强。吸电子基团 (EWG) 的放置 X 1酸增强 HB 而削弱 ChB;当 EWG 放在环上时,情况正好相反。通过选择两个单元上的适当取代基,可以在这两个键的强度之间实现近乎完美的平衡。这些键强度也反映在各种原子的化学屏蔽和直接参与每个键的原子核之间的耦合的 NMR 光谱特性中。
更新日期:2022-11-16
中文翻译:

调整氢键和硫属键之间的平衡
组装了一个络合物,它将 X 1 COOH 的羧基与其 C 原子上含有取代基的 1,2,5-硫属二唑环配对。羧基的 OH 向环的 N 原子提供一个质子,形成 OH⋯N 氢键 (HB),而其羰基 O 与 Y⋯O 硫属元素键 (ChB) 结合,其中 Y = S、Se、Te。通过将 Y 原子的尺寸从 S 扩大到 Se 再到 Te,ChB 得到加强。吸电子基团 (EWG) 的放置 X 1酸增强 HB 而削弱 ChB;当 EWG 放在环上时,情况正好相反。通过选择两个单元上的适当取代基,可以在这两个键的强度之间实现近乎完美的平衡。这些键强度也反映在各种原子的化学屏蔽和直接参与每个键的原子核之间的耦合的 NMR 光谱特性中。


























 京公网安备 11010802027423号
京公网安备 11010802027423号