当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selective TASK-1 Inhibitor with a Defined Structure–Activity Relationship Reduces Cancer Cell Proliferation and Viability
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2022-11-15 , DOI: 10.1021/acs.jmedchem.1c00378 Bárbara Arévalo 1 , Mauricio Bedoya 2, 3 , Aytug K Kiper 4 , Fernando Vergara 5 , David Ramírez 6 , Yuliet Mazola 5 , Daniel Bustos 2, 7 , Rafael Zúñiga 8, 9 , Rocio Cikutovic 8 , Angel Cayo 8 , Susanne Rinné 4 , M Teresa Ramirez-Apan 10 , Francisco V Sepúlveda 11, 12 , Oscar Cerda 13, 14 , Eduardo López-Collazo 15 , Niels Decher 4, 16 , Leandro Zúñiga 8 , Margarita Gutierrez 17 , Wendy González 5, 18
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2022-11-15 , DOI: 10.1021/acs.jmedchem.1c00378 Bárbara Arévalo 1 , Mauricio Bedoya 2, 3 , Aytug K Kiper 4 , Fernando Vergara 5 , David Ramírez 6 , Yuliet Mazola 5 , Daniel Bustos 2, 7 , Rafael Zúñiga 8, 9 , Rocio Cikutovic 8 , Angel Cayo 8 , Susanne Rinné 4 , M Teresa Ramirez-Apan 10 , Francisco V Sepúlveda 11, 12 , Oscar Cerda 13, 14 , Eduardo López-Collazo 15 , Niels Decher 4, 16 , Leandro Zúñiga 8 , Margarita Gutierrez 17 , Wendy González 5, 18
Affiliation
|
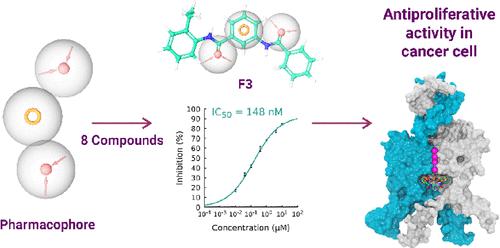
Chemical structures of selective blockers of TASK channels contain aromatic groups and amide bonds. Using this rationale, we designed and synthesized a series of compounds based on 3-benzamidobenzoic acid. These compounds block TASK-1 channels by binding to the central cavity. The most active compound is 3-benzoylamino-N-(2-ethyl-phenyl)-benzamide or F3, blocking TASK-1 with an IC50 of 148 nM, showing a reduced inhibition of TASK-3 channels and not a significant effect on different K+ channels. We identified putative F3-binding sites in the TASK-1 channel by molecular modeling studies. Mutation of seven residues to A (I118A, L122A, F125A, Q126A, L232A, I235A, and L239A) markedly decreased the F3-induced inhibition of TASK-1 channels, consistent with the molecular modeling predictions. F3 blocks cell proliferation and viability in the MCF-7 cancer cell line but not in TASK-1 knockdown MCF-7 cells, indicating that it is acting in TASK-1 channels. These results indicated that TASK-1 is necessary to drive proliferation in the MCF-7 cancer cell line.
中文翻译:

具有明确结构-活性关系的选择性 TASK-1 抑制剂可降低癌细胞增殖和活力
TASK 通道选择性阻断剂的化学结构包含芳香族基团和酰胺键。基于此原理,我们设计并合成了一系列基于 3-苯甲酰氨基苯甲酸的化合物。这些化合物通过与中央腔结合来阻断 TASK-1 通道。最活跃的化合物是 3-苯甲酰氨基 -N -(2-乙基-苯基)-苯甲酰胺或F3,它以 148 nM 的 IC 50阻断 TASK-1 ,显示出对 TASK-3 通道的抑制作用降低并且对 TASK-1 没有显着影响不同的钾+渠道。我们通过分子建模研究确定了 TASK-1 通道中推定的 F3 结合位点。七个残基突变为 A(I118A、L122A、F125A、Q126A、L232A、I235A 和 L239A)显着降低了 F3 诱导的 TASK-1 通道抑制,这与分子模型预测一致。F3阻断 MCF-7 癌细胞系中的细胞增殖和活力,但不阻断 TASK-1 敲低 MCF-7 细胞,表明它在 TASK-1 通道中起作用。这些结果表明 TASK-1 是驱动 MCF-7 癌细胞系增殖所必需的。
更新日期:2022-11-15
中文翻译:

具有明确结构-活性关系的选择性 TASK-1 抑制剂可降低癌细胞增殖和活力
TASK 通道选择性阻断剂的化学结构包含芳香族基团和酰胺键。基于此原理,我们设计并合成了一系列基于 3-苯甲酰氨基苯甲酸的化合物。这些化合物通过与中央腔结合来阻断 TASK-1 通道。最活跃的化合物是 3-苯甲酰氨基 -N -(2-乙基-苯基)-苯甲酰胺或F3,它以 148 nM 的 IC 50阻断 TASK-1 ,显示出对 TASK-3 通道的抑制作用降低并且对 TASK-1 没有显着影响不同的钾+渠道。我们通过分子建模研究确定了 TASK-1 通道中推定的 F3 结合位点。七个残基突变为 A(I118A、L122A、F125A、Q126A、L232A、I235A 和 L239A)显着降低了 F3 诱导的 TASK-1 通道抑制,这与分子模型预测一致。F3阻断 MCF-7 癌细胞系中的细胞增殖和活力,但不阻断 TASK-1 敲低 MCF-7 细胞,表明它在 TASK-1 通道中起作用。这些结果表明 TASK-1 是驱动 MCF-7 癌细胞系增殖所必需的。

























 京公网安备 11010802027423号
京公网安备 11010802027423号