当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Biased Activation Mechanism Induced by GPCR Heterodimerization: Observations from μOR/δOR Dimers
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-11-15 , DOI: 10.1021/acs.jcim.2c00962 Xin Chen 1 , Yuan Yuan 2 , Yichi Chen 1 , Jin Yu 3 , Jingzhou Wang 1 , Jianfang Chen 1 , Yanzhi Guo 1 , Xuemei Pu 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-11-15 , DOI: 10.1021/acs.jcim.2c00962 Xin Chen 1 , Yuan Yuan 2 , Yichi Chen 1 , Jin Yu 3 , Jingzhou Wang 1 , Jianfang Chen 1 , Yanzhi Guo 1 , Xuemei Pu 1
Affiliation
|
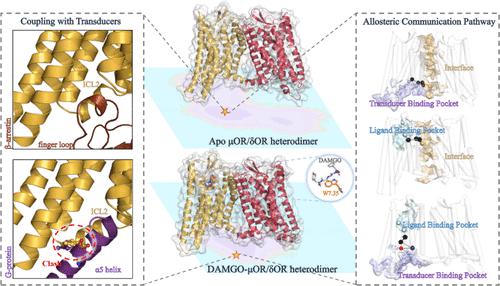
GPCRs regulate multiple intracellular signaling cascades. Biasedly activating one signaling pathway over the others provides additional clinical utility to optimize GPCR-based therapies. GPCR heterodimers possess different functions from their monomeric states, including their selectivity to different transducers. However, the biased signaling mechanism induced by the heterodimerization remains unclear. Motivated by the issue, we select an important GPCR heterodimer (μOR/δOR heterodimer) as a case and use microsecond Gaussian accelerated molecular dynamics simulation coupled with potential of mean force and protein structure network (PSN) to probe mechanisms regarding the heterodimerization-induced constitutive β-arrestin activity and efficacy change of the agonist DAMGO. The results show that only the lowest energy state of the μOR/δOR heterodimer, which adopts a slightly outward shift of TM6 and an ICL2 conformation close to the receptor core, can selectively accommodate β-arrestins. PSN further reveals important roles of H8, ICL1, and ICL2 in regulating the constitutive β-arrestin-biased activity for the apo μOR/δOR heterodimer. In addition, the heterodimerization can allosterically alter the binding mode of DAMGO mainly by means of W7.35. Consequently, DAMGO transmits the structural signal mainly through TM6 and TM7 in the dimer, rather than TM3 similar to the μOR monomer, thus changing the efficacy of DAMGO from a balanced agonist to the β-arrestin-biased one. On the other side, the binding of DAMGO to the heterodimer can stabilize μOR/δOR heterodimers through a stronger interaction of TM1/TM1 and H8/H8, accordingly enhancing the interaction of μOR with δOR and the binding affinity of the dimer to the β-arrestin. The agonist DAMGO does not change main compositions of the regulation network from the dimer interface to the transducer binding pocket of the μOR protomer, but induces an increase in the structural communication of the network, which should contribute to the enhanced β-arrestin coupling. Our observations, for the first time, reveal the molecular mechanism of the biased signaling induced by the heterodimerization for GPCRs, which should be beneficial to more comprehensively understand the GPCR bias signaling.
中文翻译:

GPCR 异二聚化诱导的偏向激活机制:μOR/δOR 二聚体的观察
GPCR 调节多个细胞内信号级联。偏向激活一种信号通路而不是其他信号通路提供了额外的临床效用来优化基于 GPCR 的疗法。GPCR 异二聚体具有与其单体状态不同的功能,包括它们对不同传感器的选择性。然而,异二聚化诱导的偏向信号传导机制仍不清楚。受此启发,我们选择一个重要的 GPCR 异二聚体 (μOR/δOR 异二聚体) 作为案例,并使用微秒高斯加速分子动力学模拟结合平均力和蛋白质结构网络 (PSN) 的潜力来探讨异二聚化诱导本构的机制激动剂 DAMGO 的 β-arrestin 活性和功效变化。结果表明,只有 μOR/δOR 异源二聚体的最低能态,采用 TM6 的轻微外移和靠近受体核心的 ICL2 构象,才能选择性地容纳 β-arrestins。PSN 进一步揭示了 H8、ICL1 和 ICL2 在调节 apo μOR/δOR 异二聚体的组成型 β-抑制蛋白偏向活性中的重要作用。此外,异二聚化主要通过 W7.35 变构改变 DAMGO 的结合模式。因此,DAMGO 主要通过二聚体中的 TM6 和 TM7 传递结构信号,而不是类似于 μOR 单体的 TM3,从而将 DAMGO 的功效从平衡激动剂转变为偏向 β-arrestin 的激动剂。另一方面,DAMGO 与异二聚体的结合可以通过 TM1/TM1 和 H8/H8 之间更强的相互作用来稳定 μOR/δOR 异二聚体,因此增强了 μOR 与 δOR 的相互作用以及二聚体与 β-arrestin 的结合亲和力。激动剂 DAMGO 不会改变调节网络的主要组成,从二聚体界面到 μOR 原体的传感器结合口袋,但会增加网络的结构通讯,这应该有助于增强 β-arrestin 耦合。我们的观察首次揭示了 GPCR 异二聚化诱导偏向信号的分子机制,这将有助于更全面地了解 GPCR 偏向信号。激动剂 DAMGO 不会改变调节网络的主要组成,从二聚体界面到 μOR 原体的传感器结合口袋,但会增加网络的结构通讯,这应该有助于增强 β-arrestin 耦合。我们的观察首次揭示了 GPCR 异二聚化诱导偏向信号的分子机制,这将有助于更全面地了解 GPCR 偏向信号。激动剂 DAMGO 不会改变调节网络的主要组成,从二聚体界面到 μOR 原体的传感器结合口袋,但会增加网络的结构通讯,这应该有助于增强 β-arrestin 耦合。我们的观察首次揭示了 GPCR 异二聚化诱导偏向信号的分子机制,这将有助于更全面地了解 GPCR 偏向信号。
更新日期:2022-11-15
中文翻译:

GPCR 异二聚化诱导的偏向激活机制:μOR/δOR 二聚体的观察
GPCR 调节多个细胞内信号级联。偏向激活一种信号通路而不是其他信号通路提供了额外的临床效用来优化基于 GPCR 的疗法。GPCR 异二聚体具有与其单体状态不同的功能,包括它们对不同传感器的选择性。然而,异二聚化诱导的偏向信号传导机制仍不清楚。受此启发,我们选择一个重要的 GPCR 异二聚体 (μOR/δOR 异二聚体) 作为案例,并使用微秒高斯加速分子动力学模拟结合平均力和蛋白质结构网络 (PSN) 的潜力来探讨异二聚化诱导本构的机制激动剂 DAMGO 的 β-arrestin 活性和功效变化。结果表明,只有 μOR/δOR 异源二聚体的最低能态,采用 TM6 的轻微外移和靠近受体核心的 ICL2 构象,才能选择性地容纳 β-arrestins。PSN 进一步揭示了 H8、ICL1 和 ICL2 在调节 apo μOR/δOR 异二聚体的组成型 β-抑制蛋白偏向活性中的重要作用。此外,异二聚化主要通过 W7.35 变构改变 DAMGO 的结合模式。因此,DAMGO 主要通过二聚体中的 TM6 和 TM7 传递结构信号,而不是类似于 μOR 单体的 TM3,从而将 DAMGO 的功效从平衡激动剂转变为偏向 β-arrestin 的激动剂。另一方面,DAMGO 与异二聚体的结合可以通过 TM1/TM1 和 H8/H8 之间更强的相互作用来稳定 μOR/δOR 异二聚体,因此增强了 μOR 与 δOR 的相互作用以及二聚体与 β-arrestin 的结合亲和力。激动剂 DAMGO 不会改变调节网络的主要组成,从二聚体界面到 μOR 原体的传感器结合口袋,但会增加网络的结构通讯,这应该有助于增强 β-arrestin 耦合。我们的观察首次揭示了 GPCR 异二聚化诱导偏向信号的分子机制,这将有助于更全面地了解 GPCR 偏向信号。激动剂 DAMGO 不会改变调节网络的主要组成,从二聚体界面到 μOR 原体的传感器结合口袋,但会增加网络的结构通讯,这应该有助于增强 β-arrestin 耦合。我们的观察首次揭示了 GPCR 异二聚化诱导偏向信号的分子机制,这将有助于更全面地了解 GPCR 偏向信号。激动剂 DAMGO 不会改变调节网络的主要组成,从二聚体界面到 μOR 原体的传感器结合口袋,但会增加网络的结构通讯,这应该有助于增强 β-arrestin 耦合。我们的观察首次揭示了 GPCR 异二聚化诱导偏向信号的分子机制,这将有助于更全面地了解 GPCR 偏向信号。



























 京公网安备 11010802027423号
京公网安备 11010802027423号