当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
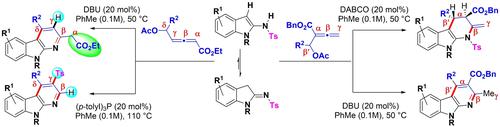
Lewis-Base Dependent (3+3) Annulations of Acetoxy Allenoates with Iminoindolines: α-Carboline Scaffolds with Varied Substituents
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2022-11-11 , DOI: 10.1002/adsc.202200997 K. C. Kumara Swamy 1 , Shubham Debnath 1 , A. Sanjeeva Kumar 1 , Sachin Chauhan 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2022-11-11 , DOI: 10.1002/adsc.202200997 K. C. Kumara Swamy 1 , Shubham Debnath 1 , A. Sanjeeva Kumar 1 , Sachin Chauhan 1
Affiliation
|
Lewis base dependent (3+3) annulations of β′/δ-acetoxy allenoates with iminoindolines offer α-carbolines with varying substituents depending on the base used as well as subtle changes in the reaction conditions. The phosphine-catalyzed annulation of δ-acetoxy allenoates with iminoindolines involves 6-exo-trig cyclization, tosyl anion elimination/trapping, and ethyl acetate elimination as key steps in delivering β-H and γ-tosyl containing α-carbolines. An unobvious elimination (by Cα-Cβ bond cleavage) of the −CH2CO2Et moiety is observed here. The same reactants under DBU catalysis offer α-carbolines that retain −CH2CO2Et moiety but are devoid of −Ts group via 6-exo-dig cyclization. The reaction of β′-acetoxy allenoate with iminoindolines is completely tertiary amine dependent; the use of DABCO affords tetrahydro-α-carbolines exclusively with excellent stereoselectivity while DBU offers substituted α-carbolines that are distinct from those using DABCO. Several control experiments and HRMS studies have been done in support of a plausible reaction mechanism.
中文翻译:

乙酰氧基联烯酸酯与亚氨基二氢吲哚的路易斯碱相关 (3+3) 环化:具有不同取代基的 α-咔啉支架
β' / δ-乙酰氧基联烯酸酯与亚氨基二氢吲哚的路易斯碱依赖性 (3+3) 环化提供具有不同取代基的α-咔啉,这取决于所用的碱以及反应条件的细微变化。膦催化的δ-乙酰氧基联烯酸与亚氨基二氢吲哚的环化涉及 6-外三角环化、甲苯磺酰基阴离子消除/捕获和乙酸乙酯消除作为传递含有α-咔啉的β -H 和γ -甲苯磺酰基的关键步骤。-CH 2 CO 2的不明显消除(通过 C α -C β键断裂)此处观察到 Et 部分。在 DBU 催化下,相同的反应物通过6-exo-dig环化提供保留了 -CH 2 CO 2 Et 部分但没有 -Ts 基团的α -咔啉。β'-乙酰氧基联烯酸酯与亚氨基二氢吲哚的反应完全依赖于叔胺;DABCO 的使用为四氢-α-咔啉提供了独有的出色立体选择性,而 DBU 提供了取代的α-咔啉,这与使用 DABCO 的那些不同。已经进行了几项控制实验和 HRMS 研究以支持合理的反应机制。
更新日期:2022-11-11
中文翻译:

乙酰氧基联烯酸酯与亚氨基二氢吲哚的路易斯碱相关 (3+3) 环化:具有不同取代基的 α-咔啉支架
β' / δ-乙酰氧基联烯酸酯与亚氨基二氢吲哚的路易斯碱依赖性 (3+3) 环化提供具有不同取代基的α-咔啉,这取决于所用的碱以及反应条件的细微变化。膦催化的δ-乙酰氧基联烯酸与亚氨基二氢吲哚的环化涉及 6-外三角环化、甲苯磺酰基阴离子消除/捕获和乙酸乙酯消除作为传递含有α-咔啉的β -H 和γ -甲苯磺酰基的关键步骤。-CH 2 CO 2的不明显消除(通过 C α -C β键断裂)此处观察到 Et 部分。在 DBU 催化下,相同的反应物通过6-exo-dig环化提供保留了 -CH 2 CO 2 Et 部分但没有 -Ts 基团的α -咔啉。β'-乙酰氧基联烯酸酯与亚氨基二氢吲哚的反应完全依赖于叔胺;DABCO 的使用为四氢-α-咔啉提供了独有的出色立体选择性,而 DBU 提供了取代的α-咔啉,这与使用 DABCO 的那些不同。已经进行了几项控制实验和 HRMS 研究以支持合理的反应机制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号