当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experimental and computational studies on rhodium-catalyzed C4(5)aryl–H activation/annulation of imidazoles with alkynes: facile synthesis of six types of N-heterocycles
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-11-10 , DOI: 10.1039/d2qo01390h Ya-Nan Tian 1 , Shihai Lv 1 , Lingyu Huang 1 , Chaoying Wen 1 , Yanyan Yang 1 , Xiangfei Kong 1 , Qiping Zhu 1 , Shiqing Li 1
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-11-10 , DOI: 10.1039/d2qo01390h Ya-Nan Tian 1 , Shihai Lv 1 , Lingyu Huang 1 , Chaoying Wen 1 , Yanyan Yang 1 , Xiangfei Kong 1 , Qiping Zhu 1 , Shiqing Li 1
Affiliation
|
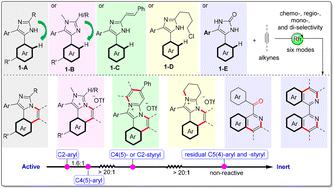
C–H annulations at N- and C2-aryls of an imidazole have been researched well, while the annulation on C4(5)-aryls especially the reactivity and site-selectivity among these aryls remains unknown. Herein, a molecular engineering strategy involving six reaction modes based on the rhodium-catalyzed C4(5)aryl–H activation/annulation of imidazoles with alkynes has been developed, giving diverse neutral/cationic N-heterocycles with broad scope (>60 examples) and high selectivity. More importantly, through a series of intramolecular competition experiments and DFT calculations, the reactivity of the peripheral C–H bonds has been studied and ranked for the first time: C2aryl–H > C4(5)aryl–H > C4(5)styryl–H/C2styryl–H > residual C4(5)aryl–H/C4(5)styryl–H. Furthermore, the remote bulky C2-subsituent is found to have a big influence on the regioselectivity of C4(5)aryl–H activation.
中文翻译:

铑催化的 C4(5) 芳基-H 活化/咪唑与炔烃环化的实验和计算研究:六种 N-杂环的简便合成
咪唑的 N- 和 C2-芳基上的 C–H 环化已得到很好的研究,而 C4(5)-芳基上的环化,尤其是这些芳基之间的反应性和位点选择性仍然未知。在此,基于铑催化的 C4(5)芳基-H 活化/咪唑与炔烃的环化,开发了一种涉及六种反应模式的分子工程策略,提供了多种具有广泛范围的中性/阳离子 N-杂环化合物(> 60 个例子)和高选择性。更重要的是,通过一系列分子内竞争实验和DFT计算,首次研究了外围C-H键的反应性,并排序为:C2 aryl –H > C4(5) aryl –H > C4(5)苯乙烯基–H/C2苯乙烯基–H > 残留的 C4(5)芳基–H/C4(5)苯乙烯基–H。此外,发现远程庞大的 C2-取代基对 C4(5)芳基-H 活化的区域选择性有很大影响。
更新日期:2022-11-10
中文翻译:

铑催化的 C4(5) 芳基-H 活化/咪唑与炔烃环化的实验和计算研究:六种 N-杂环的简便合成
咪唑的 N- 和 C2-芳基上的 C–H 环化已得到很好的研究,而 C4(5)-芳基上的环化,尤其是这些芳基之间的反应性和位点选择性仍然未知。在此,基于铑催化的 C4(5)芳基-H 活化/咪唑与炔烃的环化,开发了一种涉及六种反应模式的分子工程策略,提供了多种具有广泛范围的中性/阳离子 N-杂环化合物(> 60 个例子)和高选择性。更重要的是,通过一系列分子内竞争实验和DFT计算,首次研究了外围C-H键的反应性,并排序为:C2 aryl –H > C4(5) aryl –H > C4(5)苯乙烯基–H/C2苯乙烯基–H > 残留的 C4(5)芳基–H/C4(5)苯乙烯基–H。此外,发现远程庞大的 C2-取代基对 C4(5)芳基-H 活化的区域选择性有很大影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号