当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
NiH-Catalyzed Functionalization of Remote and Proximal Olefins: New Reactions and Innovative Strategies
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2022-11-09 , DOI: 10.1021/acs.accounts.2c00628 You Wang 1 , Yuli He 2 , Shaolin Zhu 1, 3
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2022-11-09 , DOI: 10.1021/acs.accounts.2c00628 You Wang 1 , Yuli He 2 , Shaolin Zhu 1, 3
Affiliation
|
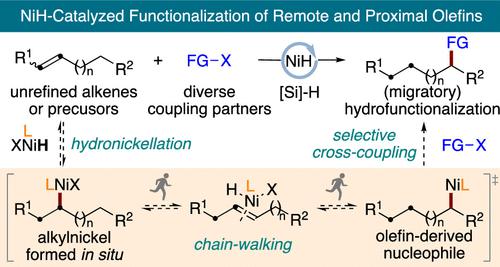
Transition metal hydride catalyzed functionalization of remote and proximal olefins has many advantages over conventional cross-coupling reactions. It avoids the separate, prior generation of stoichiometric amounts of organometallic reagents and the use of preformed organometallic reagents, which are sometimes hard to access and may compromise functional group compatibility. The migratory insertion of metal hydride complexes generated in situ into readily available alkene starting materials, the hydrometalation process, provides an attractive and straightforward route to alkyl metal intermediates, which can undergo a variety of sequential cross-coupling reactions. In particular, with the synergistic combination of chain-walking and cross-coupling chemistry of nickel, NiH-catalyzed functionalization of remote and proximal olefins has undergone particularly intense development in the past few years. This Account aims to chronicle the progress made in this arena in terms of activation modes, diverse functionalizations, and chemo-, regio-, and enantioselectivity.
中文翻译:

NiH 催化的远程和近端烯烃功能化:新反应和创新策略
远程和近端烯烃的过渡金属氢化物催化功能化与传统的交叉偶联反应相比具有许多优势。它避免了单独的、预先生成化学计量量的有机金属试剂和使用预制的有机金属试剂,这些试剂有时难以获得并且可能损害官能团相容性。原位生成的金属氢化物络合物的迁移插入加氢金属化工艺可以转化为现成的烯烃原料,为烷基金属中间体提供了一条有吸引力且直接的途径,该中间体可以进行各种连续的交叉偶联反应。特别是,随着镍的链行走和交叉偶联化学的协同组合,NiH 催化的远程和近端烯烃的功能化在过去几年中经历了特别激烈的发展。本报告旨在记录该领域在激活模式、多样化功能化以及化学选择性、区域选择性和对映选择性方面取得的进展。
更新日期:2022-11-09
中文翻译:

NiH 催化的远程和近端烯烃功能化:新反应和创新策略
远程和近端烯烃的过渡金属氢化物催化功能化与传统的交叉偶联反应相比具有许多优势。它避免了单独的、预先生成化学计量量的有机金属试剂和使用预制的有机金属试剂,这些试剂有时难以获得并且可能损害官能团相容性。原位生成的金属氢化物络合物的迁移插入加氢金属化工艺可以转化为现成的烯烃原料,为烷基金属中间体提供了一条有吸引力且直接的途径,该中间体可以进行各种连续的交叉偶联反应。特别是,随着镍的链行走和交叉偶联化学的协同组合,NiH 催化的远程和近端烯烃的功能化在过去几年中经历了特别激烈的发展。本报告旨在记录该领域在激活模式、多样化功能化以及化学选择性、区域选择性和对映选择性方面取得的进展。


























 京公网安备 11010802027423号
京公网安备 11010802027423号