当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Concise total synthesis of (±)-pileamartines A and B
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-11-08 , DOI: 10.1039/d2qo01400a Wei Xu 1, 2 , Taimin Wang 1 , Xin Zhou 1 , Lijing Fang 2 , Chen Zhang 1 , Hongbin Zhai 1, 3 , Bin Cheng 1
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-11-08 , DOI: 10.1039/d2qo01400a Wei Xu 1, 2 , Taimin Wang 1 , Xin Zhou 1 , Lijing Fang 2 , Chen Zhang 1 , Hongbin Zhai 1, 3 , Bin Cheng 1
Affiliation
|
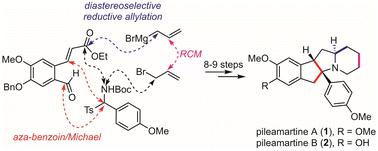
A concise total synthesis of (±)-pileamartines A and B, a pair of alkaloids sharing an unprecedented tetracyclic skeleton, was achieved in 9 and 8 linear steps, respectively. The key steps include an NHC-catalyzed tandem aza-benzoin/Michael reaction to rapidly establish the polyhydroindenopyrrole core, a diastereoselective reductive allylation of pyrrolidone, and an RCM to construct the piperidine ring.
中文翻译:

(±)-pileamartines A 和 B 的简明全合成
(±)-pileamartines A 和 B 的简明全合成,一对生物碱具有前所未有的四环骨架,分别在 9 和 8 个线性步骤中实现。关键步骤包括 NHC 催化的串联氮杂-安息香/Michael 反应以快速建立聚氢茚并吡咯核、吡咯烷酮的非对映选择性还原烯丙基化和 RCM 以构建哌啶环。
更新日期:2022-11-08
中文翻译:

(±)-pileamartines A 和 B 的简明全合成
(±)-pileamartines A 和 B 的简明全合成,一对生物碱具有前所未有的四环骨架,分别在 9 和 8 个线性步骤中实现。关键步骤包括 NHC 催化的串联氮杂-安息香/Michael 反应以快速建立聚氢茚并吡咯核、吡咯烷酮的非对映选择性还原烯丙基化和 RCM 以构建哌啶环。


























 京公网安备 11010802027423号
京公网安备 11010802027423号