当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed aza-Wacker cyclization of O-homoallyl benzimidates: expeditious access to heteroatom-rich substituted 1,3-oxazines via alkene trifunctionalization
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-11-07 , DOI: 10.1039/d2qo01233b Linlin Zhang 1 , Lin Qi 1 , Hui-Jie Du 1 , Jia-Li Liu 1 , Tong-Yang Cao 1 , Zhi-Min Yan 1 , Wei Li 1, 2 , Li-Jing Wang 1, 2
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-11-07 , DOI: 10.1039/d2qo01233b Linlin Zhang 1 , Lin Qi 1 , Hui-Jie Du 1 , Jia-Li Liu 1 , Tong-Yang Cao 1 , Zhi-Min Yan 1 , Wei Li 1, 2 , Li-Jing Wang 1, 2
Affiliation
|
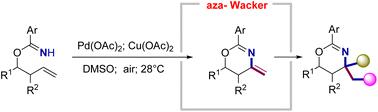
A palladium-catalyzed aza-Wacker cyclization of O-homoallyl benzimidates has been developed to afford a variety of useful 4-methylene-1,3-oxazine building blocks in moderate to good yields. The synthetic utility of the 4-methylene-1,3-oxazines is illustrated by the conversion of the exo C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond to a diverse collection of heteroatom-rich moieties through subsequent electrophilic addition enabling C–I, C–Br, C–Cl, C–O, C–N, and C–C bond formations. These tandem protocols scale well, and their products are demonstrated to be valuable frameworks for use in medicinal and biological chemistry.
C bond to a diverse collection of heteroatom-rich moieties through subsequent electrophilic addition enabling C–I, C–Br, C–Cl, C–O, C–N, and C–C bond formations. These tandem protocols scale well, and their products are demonstrated to be valuable frameworks for use in medicinal and biological chemistry.
中文翻译:

O-高烯丙基苯甲亚胺的钯催化氮杂瓦克环化:通过烯烃三官能化快速获得富含杂原子的取代1,3-恶嗪
已经开发了O-高烯丙基苯甲亚胺的钯催化氮杂-瓦克环化,以中等至良好的产率提供各种有用的 4-亚甲基-1,3-恶嗪结构单元。4-methylene-1,3-oxazines 的合成效用通过随后的亲电加成使 C-I、C-Br、C-Cl 将外C![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C 键转化为多种富含杂原子的部分来说明、C-O、C-N 和 C-C 键的形成。这些串联协议可以很好地扩展,并且它们的产品被证明是用于药物和生物化学的有价值的框架。
C 键转化为多种富含杂原子的部分来说明、C-O、C-N 和 C-C 键的形成。这些串联协议可以很好地扩展,并且它们的产品被证明是用于药物和生物化学的有价值的框架。
更新日期:2022-11-09
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond to a diverse collection of heteroatom-rich moieties through subsequent electrophilic addition enabling C–I, C–Br, C–Cl, C–O, C–N, and C–C bond formations. These tandem protocols scale well, and their products are demonstrated to be valuable frameworks for use in medicinal and biological chemistry.
C bond to a diverse collection of heteroatom-rich moieties through subsequent electrophilic addition enabling C–I, C–Br, C–Cl, C–O, C–N, and C–C bond formations. These tandem protocols scale well, and their products are demonstrated to be valuable frameworks for use in medicinal and biological chemistry.
中文翻译:

O-高烯丙基苯甲亚胺的钯催化氮杂瓦克环化:通过烯烃三官能化快速获得富含杂原子的取代1,3-恶嗪
已经开发了O-高烯丙基苯甲亚胺的钯催化氮杂-瓦克环化,以中等至良好的产率提供各种有用的 4-亚甲基-1,3-恶嗪结构单元。4-methylene-1,3-oxazines 的合成效用通过随后的亲电加成使 C-I、C-Br、C-Cl 将外C
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C 键转化为多种富含杂原子的部分来说明、C-O、C-N 和 C-C 键的形成。这些串联协议可以很好地扩展,并且它们的产品被证明是用于药物和生物化学的有价值的框架。
C 键转化为多种富含杂原子的部分来说明、C-O、C-N 和 C-C 键的形成。这些串联协议可以很好地扩展,并且它们的产品被证明是用于药物和生物化学的有价值的框架。


























 京公网安备 11010802027423号
京公网安备 11010802027423号