当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Copper-Catalyzed Asymmetric 1,6-Addition of Borylalkene-Derived Nucleophiles to para-Quinone Methides
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2022-11-04 , DOI: 10.1002/adsc.202200900 Jaesook Yun 1 , Deyuan Meng 2 , Jing He 2 , Woojin Yoon 3 , Hoseop Yun 3 , Jung Tae Han 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2022-11-04 , DOI: 10.1002/adsc.202200900 Jaesook Yun 1 , Deyuan Meng 2 , Jing He 2 , Woojin Yoon 3 , Hoseop Yun 3 , Jung Tae Han 1
Affiliation
|
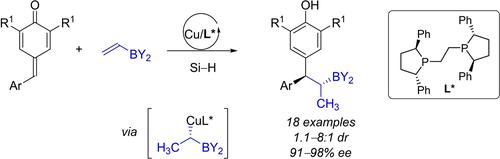
Herein, we report the catalytic 1,6-addition of chiral alkyl copper nucleophiles, in-situ generated from borylalkenes and a copper-hydride catalyst under mild conditions. Chemo- and stereoselective control was crucial in this reductive coupling process of borylalkenes and para-quinone methides, sequentially forming adjacent stereogenic centers with good diastereo- and enantioselectivity. Through the multicomponent and catalytic tandem reactions, asymmetric 1,6-adducts of p-quinone methides were efficiently synthesized.
中文翻译:

铜催化的不对称 1,6-加成硼烯衍生的亲核试剂对对醌甲基化物
在此,我们报告了手性烷基铜亲核试剂的催化 1,6-加成反应,它是在温和条件下由硼烷烃和氢化铜催化剂原位生成的。化学和立体选择性控制在硼烷烃和对苯醌甲基化物的还原偶联过程中至关重要,依次形成具有良好非对映和对映选择性的相邻立体中心。通过多组分和催化串联反应,有效地合成了对甲基苯醌的不对称 1,6-加合物。
更新日期:2022-11-04
中文翻译:

铜催化的不对称 1,6-加成硼烯衍生的亲核试剂对对醌甲基化物
在此,我们报告了手性烷基铜亲核试剂的催化 1,6-加成反应,它是在温和条件下由硼烷烃和氢化铜催化剂原位生成的。化学和立体选择性控制在硼烷烃和对苯醌甲基化物的还原偶联过程中至关重要,依次形成具有良好非对映和对映选择性的相邻立体中心。通过多组分和催化串联反应,有效地合成了对甲基苯醌的不对称 1,6-加合物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号