当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Precise control of the site selectivity in ruthenium-catalyzed C–H bond amidations using cyclic amides as powerful directing groups
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-11-05 , DOI: 10.1039/d2qo01434c Yu-Chao Yuan 1 , Qiu-Li Lu 1 , Xiao-Tong Zhu 1 , Sergio Posada-Pérez 2 , Miquel Solà 2 , Albert Poater 2 , Thierry Roisnel 3 , Rafael Gramage-Doria 3
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-11-05 , DOI: 10.1039/d2qo01434c Yu-Chao Yuan 1 , Qiu-Li Lu 1 , Xiao-Tong Zhu 1 , Sergio Posada-Pérez 2 , Miquel Solà 2 , Albert Poater 2 , Thierry Roisnel 3 , Rafael Gramage-Doria 3
Affiliation
|
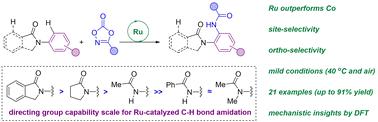
Selective C–H functionalizations aiming at the formation of new C–N bonds is of paramount importance in the context of step- and atom-economy methodologies in organic synthesis. Although the implementation of noble metal catalysts is prevalent, more benign cobalt pre-catalysts have recently appeared to be promising. However, they sometimes feature selectivity issues that limit their applicability in late-stage functionalization. Herein, we report on a highly reactive ruthenium-based catalytic system displaying excellent levels of mono-, regio- and site-selectivity by exploiting a series of biologically-relevant cyclic amides as weak directing groups. The use of dioxazolone derivatives as amidating reagents overcomes the issues encountered in the use of unstable azide derivatives for such transformations and it enables us to perform these reactions under very mild reaction conditions (air, 40 °C). Moreover, a combination of deuteration experiments and a comparative study with different types of directing groups highlights the relevance of weak amide directing groups for enabling the formation of six-membered cycloruthenate intermediates in the key elementary steps of the catalytic cycle. In addition, DFT computational calculations were carried out for the first time for studying ruthenium-catalyzed C–N bond-forming processes via C–H activation assisted by weak directing groups, thereby elucidating the origin of the regio- and site-selectivity.
中文翻译:

使用环酰胺作为强大的导向基团精确控制钌催化的 C-H 键酰胺化中的位点选择性
旨在形成新 C-N 键的选择性 C-H 功能化在有机合成中的步骤和原子经济方法的背景下至关重要。尽管贵金属催化剂的应用很普遍,但最近似乎更有希望使用更温和的钴预催化剂。然而,它们有时具有选择性问题,限制了它们在后期功能化中的适用性。在此,我们报告了一种高反应性钌基催化系统,通过利用一系列与生物学相关的环状酰胺作为弱导向基团,显示出出色的单选择性、区域选择性和位点选择性。使用二恶唑酮衍生物作为酰胺化试剂克服了使用不稳定的叠氮化物衍生物进行此类转化时遇到的问题,它使我们能够在非常温和的反应条件(空气,40 °C)下进行这些反应。此外,结合氘化实验和对不同类型导向基团的比较研究,突出了弱酰胺导向基团在催化循环的关键基本步骤中形成六元环钌酸酯中间体的相关性。此外,首次对钌催化的C-N键形成过程进行了DFT计算研究 结合氘化实验和对不同类型导向基团的比较研究,强调了弱酰胺导向基团对于在催化循环的关键基本步骤中形成六元环钌酸酯中间体的相关性。此外,首次对钌催化的C-N键形成过程进行了DFT计算研究 结合氘化实验和对不同类型导向基团的比较研究,强调了弱酰胺导向基团对于在催化循环的关键基本步骤中形成六元环钌酸酯中间体的相关性。此外,首次对钌催化的C-N键形成过程进行了DFT计算研究通过弱定向基团辅助的 C-H 活化,从而阐明了区域选择性和位点选择性的起源。
更新日期:2022-11-05
中文翻译:

使用环酰胺作为强大的导向基团精确控制钌催化的 C-H 键酰胺化中的位点选择性
旨在形成新 C-N 键的选择性 C-H 功能化在有机合成中的步骤和原子经济方法的背景下至关重要。尽管贵金属催化剂的应用很普遍,但最近似乎更有希望使用更温和的钴预催化剂。然而,它们有时具有选择性问题,限制了它们在后期功能化中的适用性。在此,我们报告了一种高反应性钌基催化系统,通过利用一系列与生物学相关的环状酰胺作为弱导向基团,显示出出色的单选择性、区域选择性和位点选择性。使用二恶唑酮衍生物作为酰胺化试剂克服了使用不稳定的叠氮化物衍生物进行此类转化时遇到的问题,它使我们能够在非常温和的反应条件(空气,40 °C)下进行这些反应。此外,结合氘化实验和对不同类型导向基团的比较研究,突出了弱酰胺导向基团在催化循环的关键基本步骤中形成六元环钌酸酯中间体的相关性。此外,首次对钌催化的C-N键形成过程进行了DFT计算研究 结合氘化实验和对不同类型导向基团的比较研究,强调了弱酰胺导向基团对于在催化循环的关键基本步骤中形成六元环钌酸酯中间体的相关性。此外,首次对钌催化的C-N键形成过程进行了DFT计算研究 结合氘化实验和对不同类型导向基团的比较研究,强调了弱酰胺导向基团对于在催化循环的关键基本步骤中形成六元环钌酸酯中间体的相关性。此外,首次对钌催化的C-N键形成过程进行了DFT计算研究通过弱定向基团辅助的 C-H 活化,从而阐明了区域选择性和位点选择性的起源。


























 京公网安备 11010802027423号
京公网安备 11010802027423号