当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
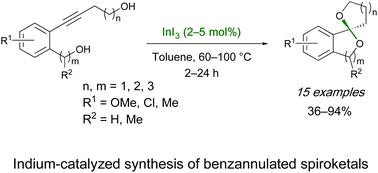
Indium-catalyzed synthesis of benzannulated spiroketals by intramolecular double hydroalkoxylation of ortho-(hydroxyalkynyl)benzyl alcohols
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-10-31 , DOI: 10.1039/d2qo01600a Raquel Pérez-Guevara 1 , Luis A. Sarandeses 1 , M. Montserrat Martínez 1 , José Pérez Sestelo 1
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-10-31 , DOI: 10.1039/d2qo01600a Raquel Pérez-Guevara 1 , Luis A. Sarandeses 1 , M. Montserrat Martínez 1 , José Pérez Sestelo 1
Affiliation
|
The novel indium-catalyzed synthesis of benzannulated spiroketals by a double intramolecular hydroalkoxylation reaction of o-(hydroxyalkynyl)benzyl alcohols is reported. The reaction proceeds under mild reaction conditions using a low catalyst loading of indium triiodide (2–5 mol%) with a variety of benzyl and homobenzyl alcohols, allowing the regioselective synthesis of a set of naturally occurring and new benzannulated spiroketals (5,5-, 5,6-, 5,7-, 6,6- and 6,7-) in good yields. The synthetic transformation involves the indium(III)-catalyzed electrophilic alkyne activation followed by the regioselective intramolecular hydroalkoxylation reaction. The method is the first example of spiroketal synthesis using main group catalysis representing an economical alternative to precious transition metals. In addition, it provides a complementary strategy for the regioselective synthesis of spiroketals and spiroaminals.
中文翻译:

邻-(羟基炔基)苄醇分子内双加氢烷氧基化铟催化合成苯环化螺缩酮
报道了通过邻-(羟基炔基)苄醇的双分子内加氢烷氧基化反应在铟催化下合成苯环化螺缩酮。该反应在温和的反应条件下进行,使用低催化剂负载的三碘化铟(2-5 mol%)与各种苄醇和高苄醇,允许区域选择性合成一组天然存在的和新的苯环化螺缩酮(5,5- , 5,6-, 5,7-, 6,6- 和 6,7-) 的良率。合成转化涉及铟(III)-催化的亲电炔烃活化,然后是区域选择性分子内加氢烷氧基化反应。该方法是使用代表贵过渡金属的经济替代品的主基团催化合成螺缩酮的第一个例子。此外,它为螺缩酮和螺胺的区域选择性合成提供了一种补充策略。
更新日期:2022-10-31
中文翻译:

邻-(羟基炔基)苄醇分子内双加氢烷氧基化铟催化合成苯环化螺缩酮
报道了通过邻-(羟基炔基)苄醇的双分子内加氢烷氧基化反应在铟催化下合成苯环化螺缩酮。该反应在温和的反应条件下进行,使用低催化剂负载的三碘化铟(2-5 mol%)与各种苄醇和高苄醇,允许区域选择性合成一组天然存在的和新的苯环化螺缩酮(5,5- , 5,6-, 5,7-, 6,6- 和 6,7-) 的良率。合成转化涉及铟(III)-催化的亲电炔烃活化,然后是区域选择性分子内加氢烷氧基化反应。该方法是使用代表贵过渡金属的经济替代品的主基团催化合成螺缩酮的第一个例子。此外,它为螺缩酮和螺胺的区域选择性合成提供了一种补充策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号