当前位置:
X-MOL 学术
›
Cancer Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Diallyl trisulfide alleviates chemotherapy sensitivity of ovarian cancer via the AMPK/SIRT1/PGC1α pathway
Cancer Science ( IF 5.7 ) Pub Date : 2022-10-30 , DOI: 10.1111/cas.15627 Zhaojun Wang 1 , Yi Yan 1 , Yijie Lou 1, 2 , Xiaoyan Huang 3 , Lijian Liu 3 , Zhuofan Weng 1 , Yusheng Cui 1 , Xinyue Wu 1 , Huijun Cai 1 , Xiaohui Chen 1 , Yunxi Ji 4
Cancer Science ( IF 5.7 ) Pub Date : 2022-10-30 , DOI: 10.1111/cas.15627 Zhaojun Wang 1 , Yi Yan 1 , Yijie Lou 1, 2 , Xiaoyan Huang 3 , Lijian Liu 3 , Zhuofan Weng 1 , Yusheng Cui 1 , Xinyue Wu 1 , Huijun Cai 1 , Xiaohui Chen 1 , Yunxi Ji 4
Affiliation
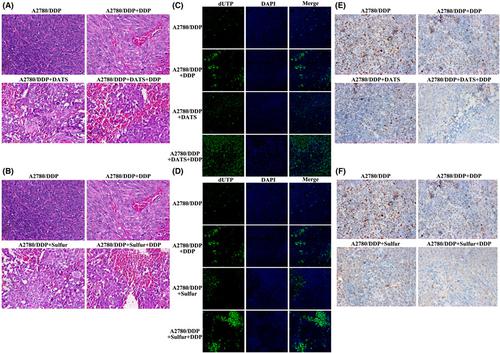
|
Platinum-based chemotherapy promotes drug resistance in ovarian cancer. We investigated the antichemoresistance characteristics of diallyl trisulfide (DATS) in cisplatin-resistant ovarian cancer cells, in vitro and in vivo. Previous preclinical studies have revealed that DATS regulates distinct hallmark cancer-signaling pathways. The cell cycle pathway is the most investigated signaling pathway in DATS. Additionally, post-DATS treatment has been found to promote proapoptotic capacity through the regulation of intrinsic and extrinsic apoptotic pathway components. In the present study, we found that treating cisplatin-sensitive and cisplatin-resistant ovarian cell lines with DATS inhibited their proliferation and reduced their IC50. It induced cell apoptosis and promoted oxidative phosphorylation through the regulation of the AMPK/SIRT1/PGC1α pathway, OXPHOS, and enhanced chemotherapy sensitivity. DATS treatment alleviated glutamine consumption in cisplatin-resistant cells. Our findings highlight the role of DATS in overcoming drug resistance in ovarian cancer in vitro and in vivo. In addition, we elucidated the role of the AMPK/SIRT1/PGC1α signaling pathway as a potential target for the treatment of drug-resistant ovarian cancer.
中文翻译:

二烯丙基三硫化物通过 AMPK/SIRT1/PGC1α 通路减轻卵巢癌化疗敏感性
基于铂的化疗促进卵巢癌的耐药性。我们在体外和体内研究了顺铂耐药卵巢癌细胞中二烯丙基三硫化物 (DATS) 的抗化学耐药特性。以前的临床前研究表明,DATS 调节不同的标志性癌症信号通路。细胞周期通路是 DATS 中研究最多的信号通路。此外,已发现 DATS 后处理可通过调节内在和外在凋亡途径成分来促进促凋亡能力。在本研究中,我们发现用 DATS 处理顺铂敏感和顺铂耐药的卵巢细胞系可抑制其增殖并降低其 IC 50。它通过调节 AMPK/SIRT1/PGC1α 通路、OXPHOS 诱导细胞凋亡并促进氧化磷酸化,增强化疗敏感性。DATS 处理减轻了顺铂耐药细胞中的谷氨酰胺消耗。我们的研究结果强调了 DATS 在体外和体内克服卵巢癌耐药性方面的作用。此外,我们阐明了 AMPK/SIRT1/PGC1α 信号通路作为治疗耐药性卵巢癌的潜在靶点的作用。
更新日期:2022-10-30
中文翻译:

二烯丙基三硫化物通过 AMPK/SIRT1/PGC1α 通路减轻卵巢癌化疗敏感性
基于铂的化疗促进卵巢癌的耐药性。我们在体外和体内研究了顺铂耐药卵巢癌细胞中二烯丙基三硫化物 (DATS) 的抗化学耐药特性。以前的临床前研究表明,DATS 调节不同的标志性癌症信号通路。细胞周期通路是 DATS 中研究最多的信号通路。此外,已发现 DATS 后处理可通过调节内在和外在凋亡途径成分来促进促凋亡能力。在本研究中,我们发现用 DATS 处理顺铂敏感和顺铂耐药的卵巢细胞系可抑制其增殖并降低其 IC 50。它通过调节 AMPK/SIRT1/PGC1α 通路、OXPHOS 诱导细胞凋亡并促进氧化磷酸化,增强化疗敏感性。DATS 处理减轻了顺铂耐药细胞中的谷氨酰胺消耗。我们的研究结果强调了 DATS 在体外和体内克服卵巢癌耐药性方面的作用。此外,我们阐明了 AMPK/SIRT1/PGC1α 信号通路作为治疗耐药性卵巢癌的潜在靶点的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号