当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lithium deposition mechanism on Si and Cu substrates in the carbonate electrolyte
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2022-10-26 , DOI: 10.1039/d2ee01833k Junhui Sun 1, 2 , Jiaying Peng 1, 2 , Terry Ring 3 , Luisa Whittaker-Brooks 4 , Juner Zhu 5 , Dimitrios Fraggedakis 6 , Jin Niu 1, 2 , Tao Gao 3 , Feng Wang 1, 2
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2022-10-26 , DOI: 10.1039/d2ee01833k Junhui Sun 1, 2 , Jiaying Peng 1, 2 , Terry Ring 3 , Luisa Whittaker-Brooks 4 , Juner Zhu 5 , Dimitrios Fraggedakis 6 , Jin Niu 1, 2 , Tao Gao 3 , Feng Wang 1, 2
Affiliation
|
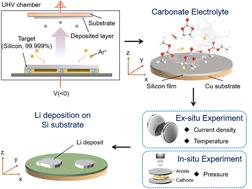
Electrochemical Li deposition occurs in fast-charging Li-ion batteries and Li metal batteries. The morphologies of the Li deposits govern the reversibility of the deposition/stripping reaction, and affect the tendency of internal-shorting, therefore determining the performance and safety of Li-ion and Li metal batteries. Many different morphologies, including hemi-sphere, granular, columnar, whisker, and dendrite, have been observed. However, how Li deposits grow into different morphologies largely remains elusive. In this work, we reveal the mechanism of Li growth on Si and Cu substrates in a commercial carbonate electrolyte by combing electron imaging, optical imaging, molecular dynamics simulation and electrochemical tests with theoretical analysis. Li growth dynamics is analyzed by comparing images of Li deposits at different currents, capacities, and temperatures. For Si substrates, a 3D-2D growth mechanism is found almost irrespective of the current density. For Cu substrates, the growth follows a 3D-2D mechanism under quasi-equilibrium conditions but transitions to a different mechanism under high current density, in which a 1D Li whisker grows out from Li islands. The tendency of whisker growth on both substrates is quantified and its dependence on current density is determined. It is revealed that all Li deposits on Cu become whiskers beyond a critical current density (Jw = 0.79 ± 0.10 mA cm−2) while Si substrates have a much larger Jw (>10 mA cm−2), leading to virtually no whisker formation. In addition, correlations between whisker growth, coulombic efficiency, and the tendency of internal shorting are determined. The nucleation and growth mechanism, as well as the transition between morphologies, are comprehensively discussed based on the results of this work and state-of-the-art findings on Li deposition.
中文翻译:

碳酸盐电解质中 Si 和 Cu 基底上的锂沉积机理
电化学锂沉积发生在快速充电的锂离子电池和锂金属电池中。锂沉积物的形态决定了沉积/剥离反应的可逆性,并影响内部短路的趋势,因此决定了锂离子和锂金属电池的性能和安全性。已经观察到许多不同的形态,包括半球形、粒状、柱状、晶须和枝晶。然而,锂沉积物如何生长成不同的形态在很大程度上仍然难以捉摸。在这项工作中,我们通过将电子成像、光学成像、分子动力学模拟和电化学测试与理论分析相结合,揭示了锂在商业碳酸盐电解质中的 Si 和 Cu 基底上生长的机制。通过比较不同电流下锂沉积物的图像来分析锂的生长动态,容量和温度。对于 Si 衬底,发现几乎与电流密度无关的 3D-2D 生长机制。对于 Cu 衬底,在准平衡条件下生长遵循 3D-2D 机制,但在高电流密度下转变为不同的机制,其中 1D Li 晶须从 Li 岛中生长出来。量化了两种基板上晶须生长的趋势,并确定了其对电流密度的依赖性。结果表明,Cu 上的所有锂沉积物都变成超过临界电流密度的晶须(其中从李岛长出一维李须。量化了两种基板上晶须生长的趋势,并确定了其对电流密度的依赖性。结果表明,Cu 上的所有锂沉积物都变成超过临界电流密度的晶须(其中从李岛长出一维李须。量化了两种基板上晶须生长的趋势,并确定了其对电流密度的依赖性。结果表明,Cu 上的所有锂沉积物都变成超过临界电流密度的晶须(J w = 0.79 ± 0.10 mA cm -2 ),而 Si 衬底具有大得多的J w (>10 mA cm -2 ),导致几乎没有晶须形成。此外,还确定了晶须生长、库仑效率和内部短路趋势之间的相关性。基于这项工作的结果和锂沉积的最新发现,全面讨论了成核和生长机制以及形态之间的转变。
更新日期:2022-10-26
中文翻译:

碳酸盐电解质中 Si 和 Cu 基底上的锂沉积机理
电化学锂沉积发生在快速充电的锂离子电池和锂金属电池中。锂沉积物的形态决定了沉积/剥离反应的可逆性,并影响内部短路的趋势,因此决定了锂离子和锂金属电池的性能和安全性。已经观察到许多不同的形态,包括半球形、粒状、柱状、晶须和枝晶。然而,锂沉积物如何生长成不同的形态在很大程度上仍然难以捉摸。在这项工作中,我们通过将电子成像、光学成像、分子动力学模拟和电化学测试与理论分析相结合,揭示了锂在商业碳酸盐电解质中的 Si 和 Cu 基底上生长的机制。通过比较不同电流下锂沉积物的图像来分析锂的生长动态,容量和温度。对于 Si 衬底,发现几乎与电流密度无关的 3D-2D 生长机制。对于 Cu 衬底,在准平衡条件下生长遵循 3D-2D 机制,但在高电流密度下转变为不同的机制,其中 1D Li 晶须从 Li 岛中生长出来。量化了两种基板上晶须生长的趋势,并确定了其对电流密度的依赖性。结果表明,Cu 上的所有锂沉积物都变成超过临界电流密度的晶须(其中从李岛长出一维李须。量化了两种基板上晶须生长的趋势,并确定了其对电流密度的依赖性。结果表明,Cu 上的所有锂沉积物都变成超过临界电流密度的晶须(其中从李岛长出一维李须。量化了两种基板上晶须生长的趋势,并确定了其对电流密度的依赖性。结果表明,Cu 上的所有锂沉积物都变成超过临界电流密度的晶须(J w = 0.79 ± 0.10 mA cm -2 ),而 Si 衬底具有大得多的J w (>10 mA cm -2 ),导致几乎没有晶须形成。此外,还确定了晶须生长、库仑效率和内部短路趋势之间的相关性。基于这项工作的结果和锂沉积的最新发现,全面讨论了成核和生长机制以及形态之间的转变。



























 京公网安备 11010802027423号
京公网安备 11010802027423号