当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
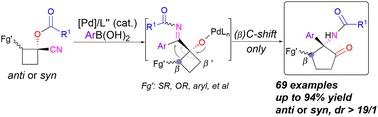
Selective synthesis of functionalized α,β-multi-substituted α-amino cyclopentanones via an α-iminol rearrangement enabled by Pd-catalyzed addition of arylboronic acids to nitriles
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-10-26 , DOI: 10.1039/d2qo01488b Qian-Qian Ma 1 , Cheng-Jing Li 1 , Wei-Wei Liao 1, 2
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-10-26 , DOI: 10.1039/d2qo01488b Qian-Qian Ma 1 , Cheng-Jing Li 1 , Wei-Wei Liao 1, 2
Affiliation
|
An efficient approach to construct α-amino cyclopentanones bearing consecutive quaternary and ternary carbon centers is described through an α-iminol rearrangement enabled by Pd-catalyzed addition of arylboronic acids to nitriles. Various α,β-multi-substituted α-amino cyclopentanones can be prepared in good to high yields under mild reaction conditions. This transformation features excellent regio-selectivity, stereospecificity, high step-economy and synthetic diversity, and offers a new protocol to prepare high-value added compounds through Pd-catalyzed addition of boronic acids to nitriles in a selective manner.
中文翻译:

Pd催化芳基硼酸与腈加成通过α-亚氨基重排选择性合成功能化α,β-多取代α-氨基环戊酮
通过 Pd 催化的芳基硼酸与腈的加成,实现了 α-亚氨基重排,描述了一种构建具有连续四元和三元碳中心的 α-氨基环戊酮的有效方法。各种α,β-多取代的α-氨基环戊酮可以在温和的反应条件下以良好至高产率制备。该转化具有优异的区域选择性、立体选择性、高步骤经济性和合成多样性,为通过钯催化硼酸选择性加成腈制备高附加值化合物提供了新的方案。
更新日期:2022-10-26
中文翻译:

Pd催化芳基硼酸与腈加成通过α-亚氨基重排选择性合成功能化α,β-多取代α-氨基环戊酮
通过 Pd 催化的芳基硼酸与腈的加成,实现了 α-亚氨基重排,描述了一种构建具有连续四元和三元碳中心的 α-氨基环戊酮的有效方法。各种α,β-多取代的α-氨基环戊酮可以在温和的反应条件下以良好至高产率制备。该转化具有优异的区域选择性、立体选择性、高步骤经济性和合成多样性,为通过钯催化硼酸选择性加成腈制备高附加值化合物提供了新的方案。



























 京公网安备 11010802027423号
京公网安备 11010802027423号