当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pillararene-Inspired Macrocycles: From Extended Pillar[n]arenes to Geminiarenes
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2022-10-20 , DOI: 10.1021/acs.accounts.2c00555 Jia-Rui Wu 1 , Gengxin Wu 1 , Ying-Wei Yang 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2022-10-20 , DOI: 10.1021/acs.accounts.2c00555 Jia-Rui Wu 1 , Gengxin Wu 1 , Ying-Wei Yang 1
Affiliation
|
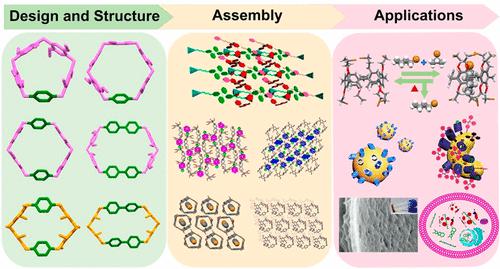
Macrocyclic compounds have been the primary tools in supramolecular chemistry since their establishment due to their innate functional features of molecular recognition and complexation. The rapid development of modern supramolecular chemistry has also significantly benefited from creating new macrocycles with distinctive geometries and properties. For instance, pillar[n]arenes (pillarenes), a relatively young generation of star macrocyclic hosts among the well-established ones (e.g., crown ethers, cyclodextrins, cucurbiturils, and calixarenes), promoted a phenomenal research hotspot all over the world in the past decade. Although the synthesis, host–guest properties, and various supramolecular functions of pillarenes have been intensively studied, many objective limitations and challenges still cannot be ignored. For example, high-level pillar[n]arenes (n > 7) usually do not possess applicable large-sized cavities due to structural folding and cannot be synthesized on a large scale because of the uncompetitive cyclization process. Furthermore, two functional groups must be covalently para-connected to each repeating phenylene unit, which severely limits their structural diversity and flexibility. In this context, we have developed a series of pillarene-inspired macrocycles (PIMs) using a versatile and modular synthetic strategy during the past few years, aiming to break through the synthetic limitations in traditional pillarenes and find new opportunities and challenges in supramolecular chemistry and beyond. Specifically, by grafting biphenyl units into the pillarene backbones, extended pillar[n]arenes with rigid and nanometer-sized cavities could be obtained with reasonable synthetic yields by selectively removing hydroxy/alkoxy substitutes on pillarene backbones, leaning pillar[6]arenes and leggero pillar[n]arenes with enhanced structural flexibility and cavity adaptability were obtained. By combining the two types of bridging modes in pillarenes and calixarenes, a smart macrocyclic receptor with two different but interconvertible conformational features, namely geminiarene, was discovered. Benefiting from the synthetic accessibility, facile functionalization, and superior host–guest properties in solution or the solid state, this new family of macrocycles has exhibited a broad range of applications, including but not limited to supramolecular assembly/gelation/polymers, pollutant detection and separation, porous organic polymers, crystalline/amorphous molecular materials, hybrid materials, and controlled drug delivery. Thus, in this Account, we summarize our research efforts on these PIMs. We first present an overview of their design and modular synthesis and a summary of their derivatization strategies. Thereafter, particular attention is paid to their structural features, supramolecular functions, and application exploration. Finally, the remaining challenges and perspectives are outlined for their future development. We hope that this Account and our works can stimulate further advances in synthetic macrocyclic chemistry and supramolecular functional systems, leading to practical applications in various research areas.
中文翻译:

柱芳烃启发的大环化合物:从扩展柱[n]芳烃到双芳烃
由于分子识别和络合的先天功能特征,大环化合物自成立以来一直是超分子化学的主要工具。现代超分子化学的快速发展也极大地受益于创造具有独特几何形状和性质的新大环化合物。例如, pillar[ n]芳烃(pillarenes)是成熟大环化合物(如冠醚、环糊精、葫芦脲和杯芳烃)中较年轻的一类星形大环化合物,在过去十年中在全球范围内成为了一个现象级的研究热点。尽管对柱烯的合成、主客体性质和各种超分子功能进行了深入研究,但许多客观限制和挑战仍然不容忽视。例如,高级柱[ n ]芳烃(n >7)由于结构折叠通常不具有适用的大尺寸空腔,并且由于无竞争的环化过程而无法大规模合成。此外,两个官能团必须共价对位-连接到每个重复的亚苯基单元,这严重限制了它们的结构多样性和灵活性。在此背景下,我们在过去几年中采用多功能和模块化的合成策略开发了一系列受柱烯启发的大环化合物(PIM),旨在突破传统柱烯的合成限制,寻找超分子化学和新的机遇和挑战。超过。具体来说,通过将联苯单元接枝到柱烯主链上,通过选择性地去除柱烯主链上的羟基/烷氧基取代基、倾斜柱 [6] 芳烃和 leggero,可以获得具有刚性和纳米尺寸空腔的扩展柱 [ n ] 芳烃,并具有合理的合成产率支柱[ n获得了结构柔韧性和空腔适应性增强的芳烃。通过结合柱烯和杯芳烃中的两种类型的桥接模式,发现了一种具有两种不同但可相互转换的构象特征的智能大环受体,即双芳烃。受益于合成的可及性、容易的功能化以及在溶液或固态下的卓越主客体特性,这个新的大环化合物家族展示了广泛的应用,包括但不限于超分子组装/凝胶化/聚合物、污染物检测和分离、多孔有机聚合物、结晶/无定形分子材料、杂化材料和受控药物输送。因此,在此帐户中,我们总结了我们对这些 PIM 的研究工作。我们首先概述了它们的设计和模块化合成,并总结了它们的衍生化策略。此后,特别关注它们的结构特征、超分子功能和应用探索。最后,概述了未来发展的剩余挑战和前景。我们希望这个帐户和我们的工作能够促进合成大环化学和超分子功能系统的进一步发展,从而在各个研究领域实现实际应用。
更新日期:2022-10-20
中文翻译:

柱芳烃启发的大环化合物:从扩展柱[n]芳烃到双芳烃
由于分子识别和络合的先天功能特征,大环化合物自成立以来一直是超分子化学的主要工具。现代超分子化学的快速发展也极大地受益于创造具有独特几何形状和性质的新大环化合物。例如, pillar[ n]芳烃(pillarenes)是成熟大环化合物(如冠醚、环糊精、葫芦脲和杯芳烃)中较年轻的一类星形大环化合物,在过去十年中在全球范围内成为了一个现象级的研究热点。尽管对柱烯的合成、主客体性质和各种超分子功能进行了深入研究,但许多客观限制和挑战仍然不容忽视。例如,高级柱[ n ]芳烃(n >7)由于结构折叠通常不具有适用的大尺寸空腔,并且由于无竞争的环化过程而无法大规模合成。此外,两个官能团必须共价对位-连接到每个重复的亚苯基单元,这严重限制了它们的结构多样性和灵活性。在此背景下,我们在过去几年中采用多功能和模块化的合成策略开发了一系列受柱烯启发的大环化合物(PIM),旨在突破传统柱烯的合成限制,寻找超分子化学和新的机遇和挑战。超过。具体来说,通过将联苯单元接枝到柱烯主链上,通过选择性地去除柱烯主链上的羟基/烷氧基取代基、倾斜柱 [6] 芳烃和 leggero,可以获得具有刚性和纳米尺寸空腔的扩展柱 [ n ] 芳烃,并具有合理的合成产率支柱[ n获得了结构柔韧性和空腔适应性增强的芳烃。通过结合柱烯和杯芳烃中的两种类型的桥接模式,发现了一种具有两种不同但可相互转换的构象特征的智能大环受体,即双芳烃。受益于合成的可及性、容易的功能化以及在溶液或固态下的卓越主客体特性,这个新的大环化合物家族展示了广泛的应用,包括但不限于超分子组装/凝胶化/聚合物、污染物检测和分离、多孔有机聚合物、结晶/无定形分子材料、杂化材料和受控药物输送。因此,在此帐户中,我们总结了我们对这些 PIM 的研究工作。我们首先概述了它们的设计和模块化合成,并总结了它们的衍生化策略。此后,特别关注它们的结构特征、超分子功能和应用探索。最后,概述了未来发展的剩余挑战和前景。我们希望这个帐户和我们的工作能够促进合成大环化学和超分子功能系统的进一步发展,从而在各个研究领域实现实际应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号