当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A trade-off between ligand and strain effects optimizes the oxygen reduction activity of Pt alloys
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2022-10-20 , DOI: 10.1039/d2ee01850k Regina M. Kluge 1 , Richard W. Haid 1 , Alexander Riss 2 , Yang Bao 2 , Knud Seufert 2 , Thorsten O. Schmidt 1 , Sebastian A. Watzele 1 , Johannes V. Barth 2 , Francesco Allegretti 2 , Willi Auwärter 2 , Federico Calle-Vallejo 3, 4, 5 , Aliaksandr S. Bandarenka 1, 6
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2022-10-20 , DOI: 10.1039/d2ee01850k Regina M. Kluge 1 , Richard W. Haid 1 , Alexander Riss 2 , Yang Bao 2 , Knud Seufert 2 , Thorsten O. Schmidt 1 , Sebastian A. Watzele 1 , Johannes V. Barth 2 , Francesco Allegretti 2 , Willi Auwärter 2 , Federico Calle-Vallejo 3, 4, 5 , Aliaksandr S. Bandarenka 1, 6
Affiliation
|
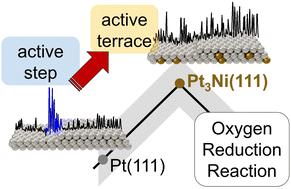
To optimize the performance of catalytic materials, it is paramount to elucidate the dependence of the chemical reactivity on the atomic arrangement of the catalyst surface. Therefore, identifying the nature of the active sites that provide optimal binding of reaction intermediates is the first step toward a rational catalyst design. In this work, we focus on the oxygen reduction reaction (ORR), an essential constituent of several energy provision and storage devices. Among the state-of-the-art ORR catalysts are platinum (Pt) and its alloys. The latter benefit from the so-called ligand and strain effects, which influence the electronic properties of the surface. Here, we “visualize” the active sites on Pt3Ni(111) in an acidic medium with a lateral resolution in the nanometre regime via an in situ technique based on electrochemical scanning tunnelling microscopy. In contrast to pure Pt, where the active sites are located at concave sites close to steps, Pt3Ni(111) terraces contain the most active centres, while steps show activity to a comparable or lesser extent. We confirm the experimental findings by a model based on alloy- and strain-sensitive generalized coordination numbers. With this model, we are also able to assess both the composition and the geometric configuration of optimal catalytic active sites on various Pt alloy catalysts. In general, the interplay of ligand effects and lattice compression resulting from the alloying of Pt with 3d transition metals (Ti, Co, Ni, Cu) gradually increases the generalized coordination number of surface Pt atoms, thereby making (111) terraces highly active. This combination of theoretical and experimental tools provides clear strategies to design more efficient Pt alloy electrocatalysts for oxygen reduction.
中文翻译:

配体和应变效应之间的权衡优化了 Pt 合金的氧还原活性
为了优化催化材料的性能,阐明化学反应性对催化剂表面原子排列的依赖性至关重要。因此,确定提供最佳反应中间体结合的活性位点的性质是合理催化剂设计的第一步。在这项工作中,我们专注于氧还原反应 (ORR),它是几种能量供应和储存装置的重要组成部分。最先进的 ORR 催化剂包括铂 (Pt) 及其合金。后者受益于所谓的配体和应变效应,这会影响表面的电子特性。在这里,我们“可视化”在酸性介质中Pt 3 Ni(111) 上的活性位点,在纳米范围内具有横向分辨率一种基于电化学扫描隧道显微镜的原位技术。与活性位点位于靠近台阶的凹形位点的纯 Pt 相比,Pt 3Ni(111)阶地包含最活跃的中心,而台阶显示出相当或较小程度的活动。我们通过基于合金和应变敏感的广义配位数的模型证实了实验结果。使用该模型,我们还能够评估各种 Pt 合金催化剂上最佳催化活性位点的组成和几何构型。一般来说,Pt 与 3d 过渡金属(Ti、Co、Ni、Cu)合金化导致的配体效应和晶格压缩的相互作用逐渐增加了表面 Pt 原子的广义配位数,从而使 (111) 阶地高度活跃。这种理论和实验工具的结合为设计更有效的氧还原铂合金电催化剂提供了明确的策略。
更新日期:2022-10-20
中文翻译:

配体和应变效应之间的权衡优化了 Pt 合金的氧还原活性
为了优化催化材料的性能,阐明化学反应性对催化剂表面原子排列的依赖性至关重要。因此,确定提供最佳反应中间体结合的活性位点的性质是合理催化剂设计的第一步。在这项工作中,我们专注于氧还原反应 (ORR),它是几种能量供应和储存装置的重要组成部分。最先进的 ORR 催化剂包括铂 (Pt) 及其合金。后者受益于所谓的配体和应变效应,这会影响表面的电子特性。在这里,我们“可视化”在酸性介质中Pt 3 Ni(111) 上的活性位点,在纳米范围内具有横向分辨率一种基于电化学扫描隧道显微镜的原位技术。与活性位点位于靠近台阶的凹形位点的纯 Pt 相比,Pt 3Ni(111)阶地包含最活跃的中心,而台阶显示出相当或较小程度的活动。我们通过基于合金和应变敏感的广义配位数的模型证实了实验结果。使用该模型,我们还能够评估各种 Pt 合金催化剂上最佳催化活性位点的组成和几何构型。一般来说,Pt 与 3d 过渡金属(Ti、Co、Ni、Cu)合金化导致的配体效应和晶格压缩的相互作用逐渐增加了表面 Pt 原子的广义配位数,从而使 (111) 阶地高度活跃。这种理论和实验工具的结合为设计更有效的氧还原铂合金电催化剂提供了明确的策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号