当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Phenanthrene-Based Polycycles by Gold(I)-Catalyzed Cyclization of Biphenyl-Embedded Trienynes
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2022-10-17 , DOI: 10.1002/adsc.202200887 Ana Milián 1 , Patricia García-García 2 , Juan J. Vaquero 2 , Roberto Sanz 3 , Manuel Ángel Fernández-Rodríguez 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2022-10-17 , DOI: 10.1002/adsc.202200887 Ana Milián 1 , Patricia García-García 2 , Juan J. Vaquero 2 , Roberto Sanz 3 , Manuel Ángel Fernández-Rodríguez 1
Affiliation
|
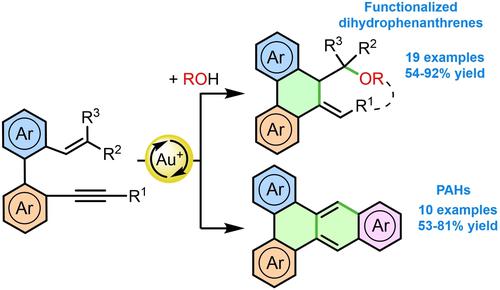
Gold(I)-catalyzed cyclization of o-alkenyl-o’-alkynylbiaryls in the presence of external or internal nucleophiles provides a straightforward access to phenanthrene-based polycycles, which are of considerable interest in materials science. Thus, their reactions with alcohols yield functionalized dihydrophenanthrenes, in a process that can also be carried out intramolecularly, to provide phenanthrene-derived heteropolycyclic compounds. Moreover, benzo[b]triphenylenes can be synthesized from o-methoxyvinyl-o’-alkynylbiaryls, in a reaction in which an (hetero)aryl substituent at the triple bond acts as an internal nucleophile.
中文翻译:

金 (I) 催化联苯嵌入三烯炔的环化反应合成菲基多环化合物
在外部或内部亲核试剂存在下,金 (I) 催化的o -烯基- o' -炔基联芳基环化提供了一种直接获得基于菲的多环化合物的途径,这在材料科学中具有相当大的意义。因此,它们与醇的反应产生功能化的二氢菲,该过程也可以在分子内进行,以提供菲衍生的杂多环化合物。此外,苯并[ b ]苯并[b]联苯可以由o-甲氧基乙烯基-o'-炔基联芳基合成,在其中三键上的(杂)芳基取代基充当内部亲核试剂的反应中。
更新日期:2022-10-17
中文翻译:

金 (I) 催化联苯嵌入三烯炔的环化反应合成菲基多环化合物
在外部或内部亲核试剂存在下,金 (I) 催化的o -烯基- o' -炔基联芳基环化提供了一种直接获得基于菲的多环化合物的途径,这在材料科学中具有相当大的意义。因此,它们与醇的反应产生功能化的二氢菲,该过程也可以在分子内进行,以提供菲衍生的杂多环化合物。此外,苯并[ b ]苯并[b]联苯可以由o-甲氧基乙烯基-o'-炔基联芳基合成,在其中三键上的(杂)芳基取代基充当内部亲核试剂的反应中。



























 京公网安备 11010802027423号
京公网安备 11010802027423号