Chem ( IF 23.5 ) Pub Date : 2022-10-04 , DOI: 10.1016/j.chempr.2022.09.012 Alexander W Schuppe 1 , Yannan Liu 1 , Elsie Gonzalez-Hurtado 2 , Yizhou Zhao 1 , Xuefeng Jiang 3 , Sebastian Ibarraran 1 , David Huang 1 , Emma Wang 1 , Jaehoo Lee 1 , J Patrick Loria 1 , Vishwa Deep Dixit 2 , Xin Li 3 , Timothy R Newhouse 1, 4
|
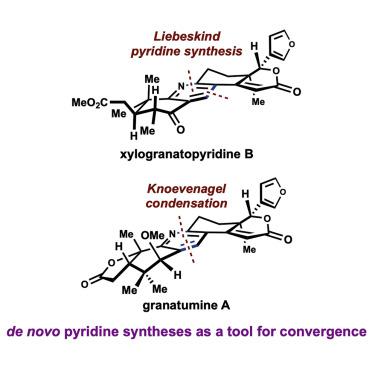
Highly substituted pyridine scaffolds are found in many biologically active natural products and therapeutics. Accordingly, numerous complementary de novo approaches to obtain differentially substituted pyridines have been disclosed. This article delineates the evolution of the synthetic strategies designed to assemble the demanding tetrasubstituted pyridine core present in the limonoid alkaloids isolated from Xylocarpus granatum, including xylogranatopyridine B, granatumine A, and related congeners. In addition, NMR calculations suggested structural misassignment of several limonoid alkaloids and predicted their C3-epimers as the correct structures, which was further validated unequivocally through chemical synthesis. The materials produced in this study were evaluated for cytotoxicity, anti-oxidant effects, anti-inflammatory action, PTP1B, and Nlrp3 inflammasome inhibition, which led to compelling anti-inflammatory activity and anti-oxidant effects being discovered.
中文翻译:

柠檬苦素生物碱的统一全合成:高度取代的吡啶支架的从头合成策略
在许多具有生物活性的天然产物和治疗药物中发现了高度取代的吡啶支架。因此,已经公开了许多获得差异取代的吡啶的互补的从头方法。本文描述了合成策略的演变,该策略旨在组装从Xylocarpus granatum中分离出的柠檬苦素生物碱(包括 xylogranatopyridine B、granatumine A 和相关同源物)中存在的要求较高的四取代吡啶核心。此外,NMR 计算表明几种柠檬苦素生物碱的结构错误,并预测它们的 C3-差向异构体为正确的结构,这通过化学合成得到了明确的进一步验证。对本研究中产生的材料的细胞毒性、抗氧化作用、抗炎作用、PTP1B 和 Nlrp3 炎性体抑制进行了评估,从而发现了引人注目的抗炎活性和抗氧化作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号