当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Noncovalent Stabilization of Radical Intermediates in the Enantioselective Hydroamination of Alkenes with Sulfonamides
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-10-05 , DOI: 10.1021/jacs.2c07099 Eve Y Xu 1 , Jacob Werth 2 , Casey B Roos 1 , Andrew J Bendelsmith 1 , Matthew S Sigman 2 , Robert R Knowles 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-10-05 , DOI: 10.1021/jacs.2c07099 Eve Y Xu 1 , Jacob Werth 2 , Casey B Roos 1 , Andrew J Bendelsmith 1 , Matthew S Sigman 2 , Robert R Knowles 1
Affiliation
|
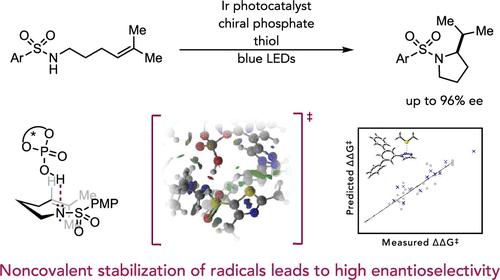
Noncovalent interactions (NCIs) are critical elements of molecular recognition in a wide variety of chemical contexts. While NCIs have been studied extensively for closed-shell molecules and ions, very little is understood about the structures and properties of NCIs involving free radical intermediates. In this report, we describe a detailed mechanistic study of the enantioselective radical hydroamination of alkenes with sulfonamides and present evidence suggesting that the basis for asymmetric induction in this process arises from attractive NCIs between a neutral sulfonamidyl radical intermediate and a chiral phosphoric acid (CPA). We describe experimental, computational, and data science-based evidence that identifies the specific radical NCIs that form the basis for the enantioselectivity. Kinetic studies support that C–N bond formation determines the enantioselectivity. Density functional theory investigations revealed the importance of both strong H-bonding between the CPA and the N-centered radical and a network of aryl-based NCIs that serve to stabilize the favored diastereomeric transition state. The contributions of these specific aryl-based NCIs to the selectivity were further confirmed through multivariate linear regression analysis by comparing the measured enantioselectivity to computed descriptors. These results highlight the power of NCIs to enable high levels of enantioselectivity in reactions involving uncharged open-shell intermediates and expand our understanding of radical–molecule interactions.
中文翻译:

自由基中间体在烯烃与磺胺类的对映选择性加氢胺化中的非共价稳定
非共价相互作用 (NCI) 是各种化学环境中分子识别的关键要素。虽然已针对闭壳分子和离子对 NCI 进行了广泛研究,但对涉及自由基中间体的 NCI 的结构和性质知之甚少。在本报告中,我们描述了烯烃与磺胺类药物的对映选择性自由基加氢胺化的详细机理研究,并提供证据表明该过程中不对称诱导的基础来自中性磺胺基自由基中间体和手性磷酸 (CPA) 之间有吸引力的 NCI . 我们描述了基于实验、计算和数据科学的证据,这些证据确定了构成对映选择性基础的特定激进 NCI。动力学研究支持 C-N 键的形成决定了对映选择性。密度泛函理论研究揭示了 CPA 和以N为中心的自由基和基于芳基的 NCI 网络,用于稳定有利的非对映体过渡态。通过将测量的对映选择性与计算的描述符进行比较,通过多元线性回归分析进一步证实了这些特定的基于芳基的 NCI 对选择性的贡献。这些结果突出了 NCI 在涉及不带电荷的开壳中间体的反应中实现高水平对映选择性的能力,并扩展了我们对自由基-分子相互作用的理解。
更新日期:2022-10-05
中文翻译:

自由基中间体在烯烃与磺胺类的对映选择性加氢胺化中的非共价稳定
非共价相互作用 (NCI) 是各种化学环境中分子识别的关键要素。虽然已针对闭壳分子和离子对 NCI 进行了广泛研究,但对涉及自由基中间体的 NCI 的结构和性质知之甚少。在本报告中,我们描述了烯烃与磺胺类药物的对映选择性自由基加氢胺化的详细机理研究,并提供证据表明该过程中不对称诱导的基础来自中性磺胺基自由基中间体和手性磷酸 (CPA) 之间有吸引力的 NCI . 我们描述了基于实验、计算和数据科学的证据,这些证据确定了构成对映选择性基础的特定激进 NCI。动力学研究支持 C-N 键的形成决定了对映选择性。密度泛函理论研究揭示了 CPA 和以N为中心的自由基和基于芳基的 NCI 网络,用于稳定有利的非对映体过渡态。通过将测量的对映选择性与计算的描述符进行比较,通过多元线性回归分析进一步证实了这些特定的基于芳基的 NCI 对选择性的贡献。这些结果突出了 NCI 在涉及不带电荷的开壳中间体的反应中实现高水平对映选择性的能力,并扩展了我们对自由基-分子相互作用的理解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号