当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solvent effects in four-component relativistic electronic structure theory based on the reference interaction-site model
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2022-10-03 , DOI: 10.1002/jcc.27009 Kodai Kanemaru 1 , Yoshihiro Watanabe 1 , Norio Yoshida 1, 2 , Haruyuki Nakano 1
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2022-10-03 , DOI: 10.1002/jcc.27009 Kodai Kanemaru 1 , Yoshihiro Watanabe 1 , Norio Yoshida 1, 2 , Haruyuki Nakano 1
Affiliation
|
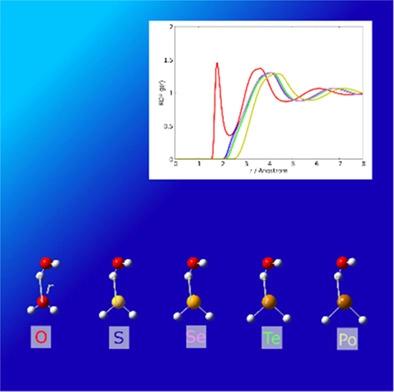
A combined method of the Dirac–Hartree–Fock (DHF) method and the reference interaction-site model (RISM) theory is reported; this is the initial implementation of the coupling of the four-component relativistic electronic structure theory and an integral equation theory of molecular liquids. In the method, the DHF and RISM equations are solved self-consistently, and therefore the electronic structure of the solute, including relativistic effects, and the solvation structure are determined simultaneously. The formulation is constructed based on the variational principle with respect to the Helmholtz energy, and analytic free energy gradients are also derived using the variational property. The method is applied to the iodine ion (I−), methyl iodide (CH3I), and hydrogen chalcogenide (H2X, where X = O–Po) in aqueous solutions, and the electronic structures of the solutes, as well as the solvation free energies and their component analysis, solvent distributions, and solute–solvent interactions, are discussed.
中文翻译:

基于参考相互作用位点模型的四组分相对论电子结构理论中的溶剂效应
报道了狄拉克-哈特里-福克 (DHF) 方法和参考交互位点模型 (RISM) 理论的组合方法;这是四分量相对论电子结构理论与分子液体积分方程理论耦合的初步实现。该方法自洽求解DHF和RISM方程,从而同时确定溶质的电子结构,包括相对论效应和溶剂化结构。该公式是基于亥姆霍兹能量的变分原理构建的,并且还使用变分特性导出了解析自由能梯度。该方法适用于碘离子 (I - )、碘甲烷 (CH 3 I) 和氢硫属化物 (H2 X,其中 X = O–Po) 在水溶液中,以及溶质的电子结构,以及溶剂化自由能及其成分分析、溶剂分布和溶质-溶剂相互作用,都进行了讨论。
更新日期:2022-10-03
中文翻译:

基于参考相互作用位点模型的四组分相对论电子结构理论中的溶剂效应
报道了狄拉克-哈特里-福克 (DHF) 方法和参考交互位点模型 (RISM) 理论的组合方法;这是四分量相对论电子结构理论与分子液体积分方程理论耦合的初步实现。该方法自洽求解DHF和RISM方程,从而同时确定溶质的电子结构,包括相对论效应和溶剂化结构。该公式是基于亥姆霍兹能量的变分原理构建的,并且还使用变分特性导出了解析自由能梯度。该方法适用于碘离子 (I - )、碘甲烷 (CH 3 I) 和氢硫属化物 (H2 X,其中 X = O–Po) 在水溶液中,以及溶质的电子结构,以及溶剂化自由能及其成分分析、溶剂分布和溶质-溶剂相互作用,都进行了讨论。



























 京公网安备 11010802027423号
京公网安备 11010802027423号