当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bulk and Interfacial Properties of the Alkane + Nitrogen System
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-09-30 , DOI: 10.1021/acs.jced.2c00533 Marcia Luna Ramirez Hincapie 1 , Arun Kumar Narayanan Nair 1 , Mohd Fuad Anwari Che Ruslan 1 , Yafan Yang 2 , Shuyu Sun 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-09-30 , DOI: 10.1021/acs.jced.2c00533 Marcia Luna Ramirez Hincapie 1 , Arun Kumar Narayanan Nair 1 , Mohd Fuad Anwari Che Ruslan 1 , Yafan Yang 2 , Shuyu Sun 1
Affiliation
|
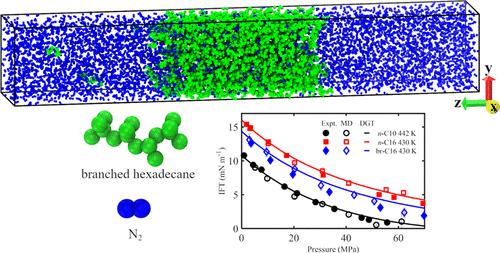
Molecular dynamics simulations were performed to investigate the bulk and interfacial properties of the alkane + N2 system at geological conditions. n-C10 + N2, n-C16 + N2, and branched C16 + N2 systems are mainly considered in this study. The simulation results were compared to theoretical modeling using the VT-PPR78 equation of state and the density gradient theory, and the results are in good agreement with the experimental findings. The density profiles of alkanes varied monotonically across the interfaces, but N2 molecules are found to enrich the interfaces. The solubility of N2 in the alkane-rich phase increases with temperature, likely due to entropic factors. This solubility also increases with pressure, likely due to energetic factors. There was no significant effect of the alkane size Nm and the chain branching on the solubilities of N2 in the alkane-rich phase. We observed a direct correlation between the solubility of N2 in the alkane-rich phase and the swelling of the alkane-rich phase. The interfacial tension (IFT) of the alkane + N2 system decreases with pressure, and this decrease is less marked at higher temperatures. For this system, the IFT decreases with temperature. Also, these IFTs increase with Nm and decrease with chain branching. Furthermore, the surface excess of N2 changes nonmonotonically as a function of pressure in the alkane + N2 system. The fact that the IFTs decreased with pressure might be explained by the positive surface excess of N2. The surface excess of N2 decreases with temperature. This explains why the decrease in the IFTs with pressure is less marked at higher temperatures. There was no significant effect of Nm and chain branching on the surface excess of N2.
中文翻译:

烷烃 + 氮气系统的体积和界面性质
进行分子动力学模拟以研究在地质条件下烷烃 + N 2系统的体积和界面性质。本研究主要考虑n -C10 + N 2、n -C16 + N 2和支链C16 + N 2体系。将模拟结果与使用VT-PPR78状态方程和密度梯度理论的理论建模进行比较,结果与实验结果吻合良好。烷烃的密度分布在界面上单调变化,但发现 N 2分子丰富了界面。N 2的溶解度在富含烷烃的相中,随着温度的升高,可能是由于熵因素。这种溶解度也随着压力的增加而增加,可能是由于能量因素。烷烃尺寸N m和支链对N 2在富烷烃相中的溶解度没有显着影响。我们观察到N 2在富烷烃相中的溶解度与富烷烃相溶胀之间的直接相关性。烷烃 + N 2体系的界面张力 (IFT) 随压力降低,并且这种降低在较高温度下不太明显。对于该系统,IFT 随温度降低。此外,这些 IFT 随N m增加并随着链支化而减少。此外,N 2的表面过量作为烷烃+N 2体系中压力的函数非单调变化。IFT 随压力下降的事实可以用 N 2的正表面过量来解释。N 2的表面过量随温度降低。这解释了为什么 IFT 随压力的降低在较高温度下不太明显。N m和链支化对N 2的表面过量没有显着影响。
更新日期:2022-09-30
中文翻译:

烷烃 + 氮气系统的体积和界面性质
进行分子动力学模拟以研究在地质条件下烷烃 + N 2系统的体积和界面性质。本研究主要考虑n -C10 + N 2、n -C16 + N 2和支链C16 + N 2体系。将模拟结果与使用VT-PPR78状态方程和密度梯度理论的理论建模进行比较,结果与实验结果吻合良好。烷烃的密度分布在界面上单调变化,但发现 N 2分子丰富了界面。N 2的溶解度在富含烷烃的相中,随着温度的升高,可能是由于熵因素。这种溶解度也随着压力的增加而增加,可能是由于能量因素。烷烃尺寸N m和支链对N 2在富烷烃相中的溶解度没有显着影响。我们观察到N 2在富烷烃相中的溶解度与富烷烃相溶胀之间的直接相关性。烷烃 + N 2体系的界面张力 (IFT) 随压力降低,并且这种降低在较高温度下不太明显。对于该系统,IFT 随温度降低。此外,这些 IFT 随N m增加并随着链支化而减少。此外,N 2的表面过量作为烷烃+N 2体系中压力的函数非单调变化。IFT 随压力下降的事实可以用 N 2的正表面过量来解释。N 2的表面过量随温度降低。这解释了为什么 IFT 随压力的降低在较高温度下不太明显。N m和链支化对N 2的表面过量没有显着影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号