当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The effects of aluminum concentration on the microstructural and electrochemical properties of lithium lanthanum zirconium oxide
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2022-09-28 , DOI: 10.1039/d2ta03676b Alexandra C. Moy 1 , Grit Häuschen 2 , Dina Fattakhova-Rohlfing 2 , Jeffrey B. Wolfenstine 3 , Martin Finsterbusch 2 , Jeff Sakamoto 1, 4
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2022-09-28 , DOI: 10.1039/d2ta03676b Alexandra C. Moy 1 , Grit Häuschen 2 , Dina Fattakhova-Rohlfing 2 , Jeffrey B. Wolfenstine 3 , Martin Finsterbusch 2 , Jeff Sakamoto 1, 4
Affiliation
|
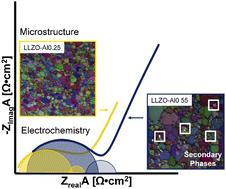
Cubic lithium lanthanum zirconium oxide (Li7−xAlxLa3Zr2O12, LLZO) garnet has gained attention as a promising next-generation electrolyte for lithium batteries due to its high ionic conductivity and chemical stability with lithium metal. The high conductivity can be achieved through doping over a range of aluminum concentrations. In this study, we hot-pressed samples to achieve <2% nominal porosity with aluminum concentrations from x = 0.25–0.55 mol to understand the effect of aluminum on microstructure and electrochemistry. It was observed that beyond the aluminum solubility limit (x = ∼0.40), resistive secondary phases formed at the grain boundaries. As a result, the percent grain boundary resistance increased from 17.6 to 41.2% for x = 0.25 and x = 0.55, respectively. Both the grain boundary and bulk activation energies remained relatively constant as the aluminum concentrations increased (∼0.44 eV and ∼0.39 eV, respectively). It was, therefore, surmised that the mobility term of the Nernst–Einstein equation was roughly independent of aluminum concentration and the major variable controlling bulk conductivity was the number of lithium charge carriers. As a result, as the aluminum concentration increased from x = 0.25 to x = 0.55 the bulk conductivity decreased from 0.56 to 0.15 mS cm−1. Following these trends of increasing grain boundary resistance and decreasing bulk conductivity with increasing aluminum concentration, x = 0.25 had the highest total conductivity (0.46 mS cm−1). We demonstrated that aluminum concentration has a significant effect on the microstructure and electrochemical properties of LLZO. We believe this work could help understand how to link processing, microstructure, and electrochemical properties to guide the manufacturing of LLZO for use in solid-state batteries.
中文翻译:

铝浓度对锂镧锆氧化物微观结构和电化学性能的影响
立方锂镧锆氧化物(Li 7− x Al x La 3 Zr 2 O 12 , LLZO)石榴石因其高离子电导率和与锂金属的化学稳定性而作为有前途的下一代锂电池电解质而备受关注。高导电性可以通过在一定范围的铝浓度上掺杂来实现。在这项研究中,我们对样品进行热压以达到 <2% 的标称孔隙率,其中铝浓度为x = 0.25–0.55 mol,以了解铝对微观结构和电化学的影响。观察到超过铝溶解度极限 ( x= ∼0.40),在晶界处形成电阻性第二相。结果,对于x = 0.25 和x = 0.55,晶界电阻百分比分别从 17.6% 增加到 41.2% 。随着铝浓度的增加,晶界和体活化能都保持相对恒定(分别为~0.44 eV和~0.39 eV)。因此,推测 Nernst-Einstein 方程的迁移率项大致与铝浓度无关,控制体积电导率的主要变量是锂电荷载流子的数量。结果,随着铝浓度从x = 0.25 增加到x = 0.55,体积电导率从 0.56 降低到 0.15 mS cm -1. 遵循随着铝浓度增加晶界电阻增加和体电导率降低的这些趋势,x = 0.25 具有最高的总电导率(0.46 mS cm -1)。我们证明了铝浓度对 LLZO 的微观结构和电化学性能有显着影响。我们相信这项工作可以帮助理解如何将加工、微观结构和电化学特性联系起来,以指导用于固态电池的 LLZO 的制造。
更新日期:2022-09-28
中文翻译:

铝浓度对锂镧锆氧化物微观结构和电化学性能的影响
立方锂镧锆氧化物(Li 7− x Al x La 3 Zr 2 O 12 , LLZO)石榴石因其高离子电导率和与锂金属的化学稳定性而作为有前途的下一代锂电池电解质而备受关注。高导电性可以通过在一定范围的铝浓度上掺杂来实现。在这项研究中,我们对样品进行热压以达到 <2% 的标称孔隙率,其中铝浓度为x = 0.25–0.55 mol,以了解铝对微观结构和电化学的影响。观察到超过铝溶解度极限 ( x= ∼0.40),在晶界处形成电阻性第二相。结果,对于x = 0.25 和x = 0.55,晶界电阻百分比分别从 17.6% 增加到 41.2% 。随着铝浓度的增加,晶界和体活化能都保持相对恒定(分别为~0.44 eV和~0.39 eV)。因此,推测 Nernst-Einstein 方程的迁移率项大致与铝浓度无关,控制体积电导率的主要变量是锂电荷载流子的数量。结果,随着铝浓度从x = 0.25 增加到x = 0.55,体积电导率从 0.56 降低到 0.15 mS cm -1. 遵循随着铝浓度增加晶界电阻增加和体电导率降低的这些趋势,x = 0.25 具有最高的总电导率(0.46 mS cm -1)。我们证明了铝浓度对 LLZO 的微观结构和电化学性能有显着影响。我们相信这项工作可以帮助理解如何将加工、微观结构和电化学特性联系起来,以指导用于固态电池的 LLZO 的制造。


























 京公网安备 11010802027423号
京公网安备 11010802027423号