当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Surface reduction in lithium- and manganese-rich layered cathodes for lithium ion batteries drives voltage decay
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2022-09-28 , DOI: 10.1039/d2ta04876k Bo Wen 1, 2 , Farheen N Sayed 3, 4 , Wesley M Dose 1, 3, 4 , Jędrzej K Morzy 1, 4, 5 , Yeonguk Son 1, 6 , Supreeth Nagendran 3 , Caterina Ducati 4, 5 , Clare P Grey 3, 4 , Michael F L De Volder 1, 4
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2022-09-28 , DOI: 10.1039/d2ta04876k Bo Wen 1, 2 , Farheen N Sayed 3, 4 , Wesley M Dose 1, 3, 4 , Jędrzej K Morzy 1, 4, 5 , Yeonguk Son 1, 6 , Supreeth Nagendran 3 , Caterina Ducati 4, 5 , Clare P Grey 3, 4 , Michael F L De Volder 1, 4
Affiliation
|
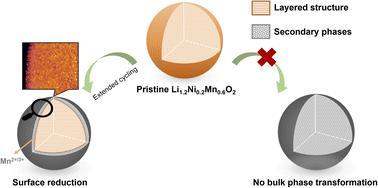
Li- and Mn-rich layered oxides (Li1.2Ni0.2Mn0.6O2) are actively pursued as high energy and sustainable alternatives to the current Li-ion battery cathodes that contain Co. However, the severe decay in discharge voltage observed in these cathodes needs to be addressed before they can find commercial applications. A few mechanisms differing in origin have been proposed to explain the voltage fade, which may be caused by differences in material composition, morphology and electrochemical testing protocols. Here, these challenges are addressed by synthesising Li1.2Ni0.2Mn0.6O2 using three different hydrothermal and solid-state approaches and studying their degradation using the same cell design and cycling protocols. The voltage fade is found to be similar under the same electrochemical testing protocols, regardless of the synthesis method. X-ray absorption near edge, extended X-ray absorption fine structure spectroscopies, and energy loss spectroscopy in a scanning transmission electron microscope indicate only minor changes in the bulk Mn oxidation state but reveal a much more reduced particle surface upon extended cycling. No spinel phase is seen via the bulk structural characterisation methods of synchrotron X-ray diffraction, 7Li magic angle spinning solid state nuclear magnetic resonance and Raman spectroscopy. Thus, the voltage fade is believed to largely result from a heavily reduced particle surface. This hypothesis is further confirmed by galvanostatic intermittent titration technique analysis, which indicates that only very small shifts in equilibrium potential take place, in contrast to the overpotential which builds up after cycling. This suggests that a major source of the voltage decay is kinetic in origin, resulting from a heavily reduced particle surface with slow Li transport.
中文翻译:

锂离子电池富锂和富锰层状阴极的表面还原驱动电压衰减
富含Li和Mn 的层状氧化物 (Li 1.2 Ni 0.2 Mn 0.6 O 2 ) 作为目前含 Co 的锂离子电池阴极的高能量和可持续替代品受到积极探索。然而,在这些材料中观察到的放电电压严重衰减在找到商业应用之前,阴极需要得到解决。已经提出了一些起源不同的机制来解释电压衰减,这可能是由材料成分、形态和电化学测试协议的差异引起的。在这里,这些挑战通过合成 Li 1.2 Ni 0.2 Mn 0.6 O 2来解决使用三种不同的水热和固态方法,并使用相同的电池设计和循环方案研究它们的降解。在相同的电化学测试协议下,无论合成方法如何,电压衰减都是相似的。边缘附近的 X 射线吸收、扩展的 X 射线吸收精细结构光谱学和扫描透射电子显微镜中的能量损失光谱表明主体 Mn 氧化态仅发生微小变化,但在延长循环后显示颗粒表面减少得多。通过同步加速器 X 射线衍射的整体结构表征方法,未发现尖晶石相, 7李魔角自旋固态核磁共振和拉曼光谱。因此,电压衰减被认为主要是由颗粒表面严重减少引起的。恒电流间歇滴定技术分析进一步证实了这一假设,这表明平衡电位仅发生非常小的变化,这与循环后建立的过电位形成对比。这表明电压衰减的一个主要来源是起源于动力学的,这是由于颗粒表面严重减少且 Li 传输缓慢所致。
更新日期:2022-09-28
中文翻译:

锂离子电池富锂和富锰层状阴极的表面还原驱动电压衰减
富含Li和Mn 的层状氧化物 (Li 1.2 Ni 0.2 Mn 0.6 O 2 ) 作为目前含 Co 的锂离子电池阴极的高能量和可持续替代品受到积极探索。然而,在这些材料中观察到的放电电压严重衰减在找到商业应用之前,阴极需要得到解决。已经提出了一些起源不同的机制来解释电压衰减,这可能是由材料成分、形态和电化学测试协议的差异引起的。在这里,这些挑战通过合成 Li 1.2 Ni 0.2 Mn 0.6 O 2来解决使用三种不同的水热和固态方法,并使用相同的电池设计和循环方案研究它们的降解。在相同的电化学测试协议下,无论合成方法如何,电压衰减都是相似的。边缘附近的 X 射线吸收、扩展的 X 射线吸收精细结构光谱学和扫描透射电子显微镜中的能量损失光谱表明主体 Mn 氧化态仅发生微小变化,但在延长循环后显示颗粒表面减少得多。通过同步加速器 X 射线衍射的整体结构表征方法,未发现尖晶石相, 7李魔角自旋固态核磁共振和拉曼光谱。因此,电压衰减被认为主要是由颗粒表面严重减少引起的。恒电流间歇滴定技术分析进一步证实了这一假设,这表明平衡电位仅发生非常小的变化,这与循环后建立的过电位形成对比。这表明电压衰减的一个主要来源是起源于动力学的,这是由于颗粒表面严重减少且 Li 传输缓慢所致。



























 京公网安备 11010802027423号
京公网安备 11010802027423号