当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The low-temperature synthesis of cation-ordered Ce–Zr-based oxide via an intermediate phase between Ce and Fe
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2022-09-27 , DOI: 10.1039/d2ta05068d Kazuto Murakami 1 , Yoko Sugawara 1 , Junki Tomita 1 , Akihiro Ishii 1 , Itaru Oikawa 1 , Hitoshi Takamura 1
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2022-09-27 , DOI: 10.1039/d2ta05068d Kazuto Murakami 1 , Yoko Sugawara 1 , Junki Tomita 1 , Akihiro Ishii 1 , Itaru Oikawa 1 , Hitoshi Takamura 1
Affiliation
|
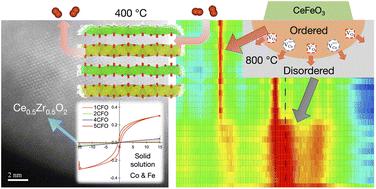
Ce0.5Zr0.5O2 is a well-known oxygen storage material used in automobiles to maintain the air–fuel ratio during operation. It has been reported that the cation ordering of Ce and Zr ions and doping with transition metals improves the oxygen storage capacity (OSC) of the Ce–Zr-based oxides. In this study, Ce–Zr-based oxides were co-doped with Co and Fe to determine their effect on the oxygen storage capacity at low temperatures. Using the Pechini method, samples of (CeZrO4)100−x–(CoFe2O4)x (xCFO; x = 1 to 5) are prepared and their solubility limit, oxygen storage capacity, and stability are clarified. The solubility limit of xCFO was found to be 5CFO. A high OSC of 440.0 μmol O2 g−1 at 400 °C was found for 5CFO, which is 13.5 times higher than the 32.5 μmol O2 g−1 found for Ce0.5Zr0.5O2 without doping. This was attributed to the low formation enthalpy of oxygen vacancy and faster surface exchange kinetics. This solid solution is stable at 400 °C and decomposes at 900 °C. The results indicate that Fe induces phase transition to a cation-ordered phase, a pyrochlore-type Ce2Zr2O7, and lowers its transition temperature to 800 °C. The acceleration of the reduction of Ce and cation diffusion due to the formation of the intermediate phase of CeFeO3 resulted in these changes. From the results of this study, it can be concluded that Fe is a key element in improving the OSC properties of the Ce–Zr-based oxide and expands the possibilities for a cation-ordered κ-Ce2Zr2O8 catalyst.
中文翻译:

通过 Ce 和 Fe 之间的中间相低温合成阳离子有序 Ce-Zr 基氧化物
Ce 0.5 Zr 0.5 O 2是一种众所周知的储氧材料,用于汽车在运行过程中保持空燃比。据报道,Ce 和 Zr 离子的阳离子排序和过渡金属的掺杂提高了 Ce-Zr 基氧化物的储氧能力 (OSC)。在这项研究中,Ce-Zr 基氧化物与 Co 和 Fe 共掺杂,以确定它们对低温下储氧能力的影响。使用 Pechini 方法, (CeZrO 4 ) 100− x –(CoFe 2 O 4 ) x ( x CFO; x= 1 至 5) 制备并阐明了它们的溶解度极限、储氧能力和稳定性。发现x CFO的溶解度极限为5CFO。对于 5CFO,在 400 °C 下发现了440.0 μmol O 2 g -1的高 OSC,比未掺杂的 Ce 0.5 Zr 0.5 O 2的 32.5 μmol O 2 g -1高 13.5 倍。这归因于氧空位的低形成焓和更快的表面交换动力学。该固溶体在 400 °C 下稳定,在 900 °C 下分解。结果表明,Fe 诱导相变到阳离子有序相,即烧绿石型 Ce 2 Zr 2O 7,并降低其转变温度至800°C。由于CeFeO 3中间相的形成加速了Ce的还原和阳离子扩散导致了这些变化。从这项研究的结果可以得出结论,Fe 是改善 Ce-Zr 基氧化物的 OSC 性能的关键元素,并扩大了阳离子有序 κ-Ce 2 Zr 2 O 8催化剂的可能性。
更新日期:2022-09-27
中文翻译:

通过 Ce 和 Fe 之间的中间相低温合成阳离子有序 Ce-Zr 基氧化物
Ce 0.5 Zr 0.5 O 2是一种众所周知的储氧材料,用于汽车在运行过程中保持空燃比。据报道,Ce 和 Zr 离子的阳离子排序和过渡金属的掺杂提高了 Ce-Zr 基氧化物的储氧能力 (OSC)。在这项研究中,Ce-Zr 基氧化物与 Co 和 Fe 共掺杂,以确定它们对低温下储氧能力的影响。使用 Pechini 方法, (CeZrO 4 ) 100− x –(CoFe 2 O 4 ) x ( x CFO; x= 1 至 5) 制备并阐明了它们的溶解度极限、储氧能力和稳定性。发现x CFO的溶解度极限为5CFO。对于 5CFO,在 400 °C 下发现了440.0 μmol O 2 g -1的高 OSC,比未掺杂的 Ce 0.5 Zr 0.5 O 2的 32.5 μmol O 2 g -1高 13.5 倍。这归因于氧空位的低形成焓和更快的表面交换动力学。该固溶体在 400 °C 下稳定,在 900 °C 下分解。结果表明,Fe 诱导相变到阳离子有序相,即烧绿石型 Ce 2 Zr 2O 7,并降低其转变温度至800°C。由于CeFeO 3中间相的形成加速了Ce的还原和阳离子扩散导致了这些变化。从这项研究的结果可以得出结论,Fe 是改善 Ce-Zr 基氧化物的 OSC 性能的关键元素,并扩大了阳离子有序 κ-Ce 2 Zr 2 O 8催化剂的可能性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号