Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2022-09-26 , DOI: 10.1016/j.jhazmat.2022.130069 Shiwei Xie 1 , Chang Li 1 , Hui Zhan 1 , Wei Shao 1 , Yuanxin Zhao 2 , Peng Liu 3 , Peng Liao 2
|
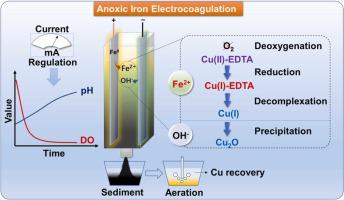
Fe-based replacement and precipitation are promising methods for removal of copper ethylenediaminetetraacetic acid (Cu(II)-EDTA) but are limited by the necessity of controlling pH and dissolved oxygen. The details of the decomplexation mechanism also remain unclear. The present work investigated an anoxic iron electrocoagulation process capable of automatically modulating anoxic conditions and solution pH during exposure to air and thus promoting the rapid and thorough decomplexation of Cu(II)-EDTA. Dissolved Fe (II), rather than Fe(II)-bearing minerals, was found to be primarily responsible for the reduction of Cu(II)-EDTA to Cu(I)-EDTA and for the subsequent replacement reaction to generate free Cu(I) ions within the initial pH range of 2–7. The Cu(I) was primarily precipitated as Cu2O on the surface of green rust and magnetite as the pH was increased. The aeration of these Fe-containing precipitates released free Cu(I) ions instead of chelated Cu into solution, allowing for recycling of the Cu. This release of Cu(I) was likely induced by the pH decrease during aeration. This study provides important insights regarding the reductive decomplexation of chelated Cu(II) and the recovery of Cu via anoxic iron electrocoagulation, which is a promising green approach to recycling Cu from wastewater.
中文翻译:

缺氧铁电凝自动调节溶解氧和 pH 值,以实现 Cu(II)-EDTA 的快速还原解络和沉淀:溶解 Fe(II) 的关键作用
铁基置换和沉淀是去除乙二胺四乙酸铜 (Cu(II)-EDTA) 的有前景的方法,但受限于控制 pH 值和溶解氧的必要性。解络合机制的细节也仍不清楚。本工作研究了一种缺氧铁电凝工艺,该工艺能够在暴露于空气期间自动调节缺氧条件和溶液 pH 值,从而促进 Cu(II)-EDTA 的快速彻底解络。发现溶解的 Fe (II),而不是含 Fe (II) 的矿物,主要负责将 Cu(II)-EDTA 还原为 Cu(I)-EDTA 以及随后的置换反应生成游离 Cu( I) 初始 pH 值范围为 2-7 的离子。Cu(I)主要以Cu 2的形式沉淀随着 pH 值的增加,绿锈和磁铁矿表面上的 O。这些含铁沉淀的曝气将游离的 Cu(I) 离子而不是螯合的 Cu 释放到溶液中,从而允许回收 Cu。这种 Cu(I) 的释放可能是由曝气过程中 pH 值降低引起的。本研究提供了关于螯合 Cu(II) 的还原解络和通过缺氧铁电凝聚回收 Cu 的重要见解,这是一种从废水中回收 Cu 的有前途的绿色方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号