当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Applying the HSAB design principle to the 3.5 V-class all-solid-state Li-ion batteries with a chloride electrolyte
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2022-09-26 , DOI: 10.1039/d2ta05152d Naoto Tanibata 1 , Shuta Takimoto 1 , Shin Aizu 1 , Hayami Takeda 1 , Masanobu Nakayama 1
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2022-09-26 , DOI: 10.1039/d2ta05152d Naoto Tanibata 1 , Shuta Takimoto 1 , Shin Aizu 1 , Hayami Takeda 1 , Masanobu Nakayama 1
Affiliation
|
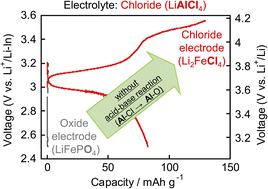
All-solid-state Li-ion batteries are expected to be the next generation of batteries with a high energy density and safety. However, for Li-ion batteries to endure high-voltage operations, the decomposition of solid electrolytes must be suppressed first. A high potential at the cathode tends to promote battery degradation because of the oxidation of the cathode electrolyte. This study aims to achieve the high-potential operation of all-solid-state batteries using LiAlCl4 as a chloride electrolyte with a high oxidation resistance. However, batteries with commonly used oxide electrodes (e.g., LiFePO4) exhibit low capacity (∼0.5 mA h g−1), despite having working potentials less than the oxidation potential of LiAlCl4. First-principles calculations and 27Al MAS-NMR measurements suggest that acid–base reactions based on the hard and soft acid–base (HSAB) rule occur between the electrode and the electrolyte. In contrast, a high voltage of ∼3.65 V (vs. Li+/Li) and high-capacity utilisation (reversible capacity ∼100 mA h g−1) are observed at room temperature by combining the same chloride electrode (Li2FeCl4) without side reactions between these chlorides. These results indicate that material design based on the HSAB rule is also instructive when considering electrode/electrolyte material combinations, which realizes a 3.5 V-class all-solid-state Li-ion battery.
中文翻译:

将 HSAB 设计原理应用于具有氯化物电解质的 3.5 V 级全固态锂离子电池
全固态锂离子电池有望成为具有高能量密度和安全性的下一代电池。然而,为了使锂离子电池能够承受高压操作,必须首先抑制固体电解质的分解。由于阴极电解质的氧化,阴极处的高电位趋于促进电池退化。本研究旨在使用 LiAlCl 4作为具有高抗氧化性的氯化物电解质,实现全固态电池的高电位运行。然而,具有常用氧化物电极(例如,LiFePO 4)的电池表现出低容量(~0.5 mA hg -1),尽管其工作电位低于 LiAlCl 4的氧化电位. 第一性原理计算和27 Al MAS-NMR 测量表明,基于硬酸碱和软酸碱 (HSAB) 规则的酸碱反应发生在电极和电解质之间。相反,通过组合相同的氯化物电极(Li 2 FeCl 4),在室温下观察到~3.65 V(相对于Li + /Li)的高电压和高容量利用率(可逆容量~100 mA hg -1 )这些氯化物之间没有副反应。这些结果表明,在考虑电极/电解质材料组合时,基于 HSAB 规则的材料设计也具有指导意义,可实现 3.5 V 级全固态锂离子电池。
更新日期:2022-09-30
中文翻译:

将 HSAB 设计原理应用于具有氯化物电解质的 3.5 V 级全固态锂离子电池
全固态锂离子电池有望成为具有高能量密度和安全性的下一代电池。然而,为了使锂离子电池能够承受高压操作,必须首先抑制固体电解质的分解。由于阴极电解质的氧化,阴极处的高电位趋于促进电池退化。本研究旨在使用 LiAlCl 4作为具有高抗氧化性的氯化物电解质,实现全固态电池的高电位运行。然而,具有常用氧化物电极(例如,LiFePO 4)的电池表现出低容量(~0.5 mA hg -1),尽管其工作电位低于 LiAlCl 4的氧化电位. 第一性原理计算和27 Al MAS-NMR 测量表明,基于硬酸碱和软酸碱 (HSAB) 规则的酸碱反应发生在电极和电解质之间。相反,通过组合相同的氯化物电极(Li 2 FeCl 4),在室温下观察到~3.65 V(相对于Li + /Li)的高电压和高容量利用率(可逆容量~100 mA hg -1 )这些氯化物之间没有副反应。这些结果表明,在考虑电极/电解质材料组合时,基于 HSAB 规则的材料设计也具有指导意义,可实现 3.5 V 级全固态锂离子电池。



























 京公网安备 11010802027423号
京公网安备 11010802027423号