Journal of Advanced Research ( IF 10.7 ) Pub Date : 2022-09-24 , DOI: 10.1016/j.jare.2022.09.007 Ying Zhu 1 , Anni Wang 2 , Shuya Zhang 2 , Jisu Kim 2 , Jiaxuan Xia 2 , Fengxue Zhang 3 , Dan Wang 4 , Qi Wang 5 , Jianxin Wang 6
|
Introduction
Inherent or acquired resistance to paclitaxel (PTX) is a pivotal challenge for chemotherapy treatment of multidrug-resistant (MDR) breast cancer. Although various targeted drug-delivery systems, including nanoparticles and liposomes, are effective for MDR cancer treatment, their efficacy is restricted by immunosuppressive tumor microenvironment (TME).
Methods
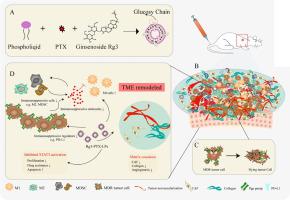
Ginsenosides Rg3 was used to formulate unique Rg3-based liposomes loaded with PTX to establish Rg3-PTX-LPs, which were prepared by the thin-film hydration method. The stability of the Rg3-PTX-LPs was evaluated by particle size analysis through dynamic light scattering. The active targeting effect of Rg3-based liposomes was examined in an MCF-7/T xenograft model by an in a vivo imaging system. To evaluate the antitumor activity and mechanism of Rg3-PTX-LP, MTT, apoptosis assays, TAM regulation, and TME remodeling were performed in MCF-7/T cells in vitro and in vivo.
Results
Rg3-PTX-LPs could specifically distribute to MCF7/T cancer cells and TME simultaneously, mainly through the recognition of GLUT-1. The drug resistance reversing capability and in vivo antitumor effect of Rg3-PTX-LPs were significantly improved compared with conventional cholesterol liposomes. The TME remodeling mechanisms of Rg3-PTX-LPs included inhibiting IL-6/STAT3/p-STAT3 pathway activation to repolarize protumor M2 macrophages to antitumor M1 phenotype, suppressing myeloid-derived suppressor cells (MDSCs), decreasing tumor-associated fibroblasts (TAFs) and collagen fibers in TME, and promoting apoptosis of tumor cells. Hence, through the dual effects of targeting tumor cells and TME remodeling, Rg3-PTX-LPs achieved a high tumor inhibition rate of 90.3%.
Conclusion
Our multifunctional Rg3-based liposome developed in the present study offered a promising strategy for rescuing the drug resistance tumor treatment.
中文翻译:

负载紫杉醇的人参皂苷 Rg3 脂质体通过肿瘤微环境和癌细胞的双重靶向治疗耐药癌症
介绍
对紫杉醇 (PTX) 的固有或获得性耐药是多重耐药 (MDR) 乳腺癌化疗的关键挑战。尽管包括纳米颗粒和脂质体在内的各种靶向药物递送系统对于MDR癌症治疗有效,但其疗效受到免疫抑制肿瘤微环境(TME)的限制。
方法
采用人参皂苷Rg3配制独特的基于Rg3的负载PTX的脂质体,建立Rg3-PTX-LPs,并通过薄膜水合法制备。通过动态光散射进行粒度分析来评估 Rg3-PTX-LP 的稳定性。通过体内成像系统在 MCF-7/T 异种移植模型中检查基于 Rg3 的脂质体的主动靶向效果。为了评估 Rg3-PTX-LP 的抗肿瘤活性和机制,在体外和体内的 MCF-7/T 细胞中进行了 MTT、细胞凋亡测定、TAM 调节和 TME 重塑。
结果
Rg3-PTX-LPs可以同时特异性分布到MCF7/T癌细胞和TME,主要是通过识别GLUT-1。与常规胆固醇脂质体相比,Rg3-PTX-LPs的耐药逆转能力和体内抗肿瘤作用显着提高。Rg3-PTX-LPs 的 TME 重塑机制包括抑制 IL-6/STAT3/p-STAT3 通路激活,使促肿瘤 M2 巨噬细胞复极化为抗肿瘤 M1 表型,抑制骨髓源性抑制细胞 (MDSC),减少肿瘤相关成纤维细胞 (TAF) )和TME中的胶原纤维,促进肿瘤细胞凋亡。因此,通过靶向肿瘤细胞和TME重塑的双重作用,Rg3-PTX-LPs实现了高达90.3%的肿瘤抑制率。
结论
我们在本研究中开发的基于 Rg3 的多功能脂质体为挽救耐药肿瘤治疗提供了一种有前途的策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号