Journal of Hepatology ( IF 25.7 ) Pub Date : 2022-09-22 , DOI: 10.1016/j.jhep.2022.09.009 Andrew Fagan 1 , Edith A Gavis 1 , Mary Leslie Gallagher 1 , Travis Mousel 1 , Brian Davis 1 , Puneet Puri 1 , Richard K Sterling 1 , Velimir A Luketic 1 , Hannah Lee 1 , Scott C Matherly 1 , Arun J Sanyal 1 , R Todd Stravitz 1 , Vaishali Patel 1 , Mohammad S Siddiqui 1 , Amon Asgharpour 1 , Michael Fuchs 1 , Leroy Thacker 2 , Jasmohan S Bajaj 1
|
Background & Aims
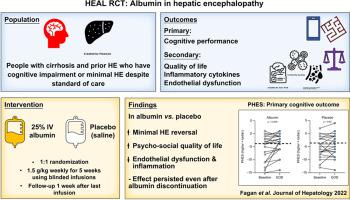
Even after recovery from overt hepatic encephalopathy (HE), minimal HE (MHE), which impairs quality of life (QoL), can persist. A double-blind, placebo-controlled randomized clinical trial was performed to determine the impact of albumin vs. saline on MHE and QoL in individuals with prior HE already on standard of care.
Methods
Outpatients with cirrhosis and prior HE, MHE and hypoalbuminemia already on treatment for HE were included. Patients on regular IV albumin infusions were excluded. Participants were randomized 1:1 to receive either weekly infusions of 25% IV albumin 1.5 g/kg or saline over 5 weeks. MHE was defined using either psychometric hepatic encephalopathy score (PHES), Stroop or critical clicker frequency. MHE, QoL (based on sickness impact profile [SIP] total, physical, psychosocial domain) and serum markers (inflammation, endothelial dysfunction, and ischemia-modified albumin) were compared between baseline, the final infusion visit (end-of-drug [EOD]) and 1-week post final infusion (end-of-study [EOS]).
Results
Forty-eight (24/group) participants were randomized and balanced (including by HE medication use) at baseline. Adverse events were similar, with MELD and ammonia remaining stable between/within groups. Albumin levels increased and ischemia-modified albumin decreased only in the albumin group at EOD and EOS vs. baseline. PHES and Stroop MHE reversal and improvement were greater in the albumin group at EOD and persisted at EOS. SIP total and psychosocial, but not physical, domain improved only in the albumin group at EOD and EOS vs. baseline. A significant reduction in IL-1β and endothelial dysfunction markers was also observed in the albumin group.
Conclusion
In a double-blind, placebo-controlled trial of outpatients with cirrhosis, prior HE and current MHE, albumin infusions were associated with improved cognitive function and psychosocial QoL, likely due to amelioration of endothelial dysfunction.
Clinical trials registration
www.clinicaltrials.gov NCT03585257.
Impact and implications
Even after recovery from overt hepatic encephalopathy (HE), minimal HE (MHE), which impairs quality of life, can persist. We found that intravenous albumin infusions were associated with improved cognitive function and psychosocial quality of life, likely owing to amelioration of endothelial dysfunction, compared to placebo in outpatients with prior HE and current MHE. In patients who continue to demonstrate cognitive dysfunction and impaired quality of life despite standard of care therapy for HE, albumin infusions could be considered if these results are validated.
中文翻译:

门诊肝性脑病患者白蛋白的双盲随机安慰剂对照试验:HEAL 研究
背景与目标
即使在从明显的肝性脑病 (HE) 中恢复后,损害生活质量 (QoL) 的轻微肝性脑病 (MHE) 也会持续存在。进行了一项双盲、安慰剂对照的随机临床试验,以确定白蛋白与盐水对既往 HE 已经接受标准护理的个体的 MHE 和 QoL的影响。
方法
门诊肝硬化和既往 HE、MHE 和已经接受 HE 治疗的低白蛋白血症患者均包括在内。定期静脉输注白蛋白的患者被排除在外。参与者以 1:1 的比例随机分配,在 5 周内接受每周输注 25% IV 白蛋白 1.5 g/kg 或生理盐水。MHE 是使用心理测量肝性脑病评分 (PHES)、Stroop 或临界点击频率来定义的。在基线、最终输液访视(药物结束 [ EOD]) 和最后一次输注后 1 周(研究结束 [EOS])。
结果
四十八名(24 名/组)参与者在基线时被随机分配和平衡(包括通过 HE 药物使用)。不良事件相似,MELD 和氨在组间/组内保持稳定。与基线相比,在 EOD 和 EOS 时白蛋白水平增加,而缺血修饰白蛋白仅在白蛋白组中减少。PHES 和 Stroop MHE 逆转和改善在 EOD 时白蛋白组更大,并在 EOS 时持续存在。SIP 总体和社会心理而非身体领域仅在 EOD 和 EOS与基线时的白蛋白组中有所改善。在白蛋白组中也观察到 IL-1β 和内皮功能障碍标志物显着减少。
结论
在一项针对门诊肝硬化、既往 HE 和当前 MHE 患者的双盲、安慰剂对照试验中,输注白蛋白与改善认知功能和社会心理 QoL 相关,这可能是由于内皮功能障碍的改善。
临床试验注册
www.clinicaltrials.gov NCT03585257。
影响和启示
即使在从明显的肝性脑病 (HE) 中恢复后,损害生活质量的轻微 HE (MHE) 也会持续存在。我们发现,与既往 HE 和当前 MHE 的门诊患者的安慰剂相比,静脉输注白蛋白与认知功能和社会心理质量的改善有关,这可能是由于内皮功能障碍的改善。尽管对 HE 进行了标准护理治疗,但仍继续表现出认知功能障碍和生活质量受损的患者,如果这些结果得到验证,则可以考虑输注白蛋白。


























 京公网安备 11010802027423号
京公网安备 11010802027423号