Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2022-09-21 , DOI: 10.1016/j.bmcl.2022.128995 Akanksha Jain 1 , Anuj Kumar 1 , R Vasumathy 2 , Suresh Subramanian 1 , H D Sarma 3 , Drishty Satpati 4
|
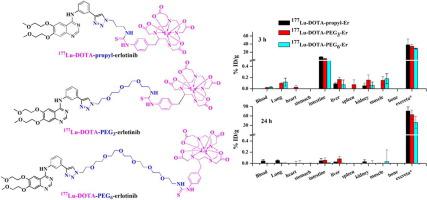
Erlotinib is a first generation epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) which was granted Food and Drug administration (FDA) approval for treatment of patients with locally advanced or metastatic NSCLC. The present study aimed at development of radiolabeled erlotinib variants as tyrosine kinase inhibitors. Three DOTA-erlotinib conjugates were prepared for radiolabeling with 177Lu. The terminal alkyne group of erlotinib was modified by performing Cu-catalyzed click chemistry and three different linkers were introduced which were then conjugated to the chelator, DOTA. The DOTA-erlotinib conjugates were characterized by 1H NMR and ESI-MS. 177Lu-DOTA-erlotinib complexes were characterized using natLu-DOTA-erlotinib conjugates. The 177Lu-complexes exhibited high in vitro stability in human serum up to 48 h. They were highly hydrophilic in nature as observed from their log Po/w values (177Lu-DOTA-propyl-Er: −2.5 ± 0.1; 177Lu-DOTA-PEG3-Er: −3.0 ± 0.1; 177Lu-DOTA-PEG6-Er: −3.3 ± 0.1). The MTT assay in A431 human epidermoid carcinoma cell lines indicates that the chemical modification at the terminal alkyne group of the erlotinib molecule does not have significant effect on its TKI property. Biodistribution studies in normal Swiss mice demonstrated fast clearance and excretion of 177Lu-labeled erlotinib complexes. These studies indicate that erlotinib variants with hydrophobic pharmacokinetic modifiers/chelators may enhance the retention of 177Lu-labeled complexes in blood thereby increasing the probability to reach EGFR-expressing tumor.
中文翻译:

放射性标记厄洛替尼类似物的制备及接头作用分析
厄洛替尼是第一代表皮生长因子受体酪氨酸激酶抑制剂(EGFR-TKI),已获得美国食品和药物管理局(FDA)批准用于治疗局部晚期或转移性非小细胞肺癌患者。本研究旨在开发放射性标记的厄洛替尼变体作为酪氨酸激酶抑制剂。制备了三种 DOTA-厄洛替尼偶联物,用于用177 Lu 进行放射性标记。厄洛替尼的末端炔基通过铜催化的点击化学进行修饰,并引入了三种不同的接头,然后将其与螯合剂 DOTA 结合。DOTA-厄洛替尼偶联物通过1 H NMR 和 ESI-MS 进行表征。使用nat表征了177 个Lu-DOTA-厄洛替尼复合物Lu-DOTA-厄洛替尼偶联物。177 个Lu 复合物在人血清中的体外稳定性长达 48 小时。从它们的 log P o/w值观察到,它们本质上是高度亲水的(177 Lu-DOTA-丙基-Er:-2.5 ± 0.1;177 Lu-DOTA-PEG 3 -Er:-3.0 ± 0.1;177 Lu-DOTA -PEG 6 -Er:-3.3 ± 0.1)。A431人表皮样癌细胞系中的MTT测定表明,厄洛替尼分子末端炔基的化学修饰对其TKI性质没有显着影响。在正常瑞士小鼠中进行的生物分布研究表明,177的快速清除和排泄Lu标记的厄洛替尼复合物。这些研究表明,具有疏水性药代动力学调节剂/螯合剂的厄洛替尼变体可以增强177 Lu 标记的复合物在血液中的保留,从而增加到达表达 EGFR 的肿瘤的可能性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号