当前位置:
X-MOL 学术
›
Chem. Eng. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adsorptive Removal of Daunorubicin from Water by Graphene Oxide, Activated Carbon, and Multiwalled Carbon Nanotubes: Equilibrium and Kinetic Studies
Chemical Engineering & Technology ( IF 2.1 ) Pub Date : 2022-09-20 , DOI: 10.1002/ceat.202200326 Atefeh Ghodrati 1 , Javad Rahbar Shahrouzi 1 , Ramin Nemati 1 , Neda Pourjafari 2
Chemical Engineering & Technology ( IF 2.1 ) Pub Date : 2022-09-20 , DOI: 10.1002/ceat.202200326 Atefeh Ghodrati 1 , Javad Rahbar Shahrouzi 1 , Ramin Nemati 1 , Neda Pourjafari 2
Affiliation
|
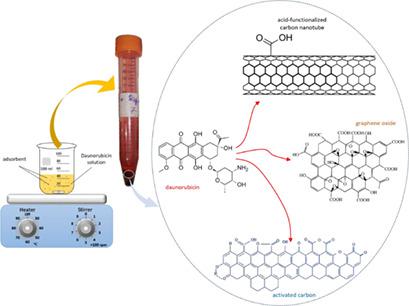
The adsorption of anticancer drug daunorubicin from aqueous solution by carbonaceous adsorbents was investigated. The adsorption performances of graphene oxide (GO), activated carbon (AC), and multiwalled carbon nanotubes (MWCNTs) were compared. The effects of solution pH, contact time, and initial daunorubicin concentration on the removal of daunorubicin were explored. Isotherm and kinetic models were applied to predict the efficiency of the adsorbents. The major functional groups of the adsorbents were identified by FTIR spectroscopy. In alkaline medium, adsorption increases with increasing pH value. With increasing initial concentration, adsorption capacity increases and removal efficiency decreases. Kinetic studies showed that the pseudo-second-order model describes adsorption onto GO well, whereas the intraparticle diffusion model gives the best fits for AC and MWCNTs. The adsorption isotherm fits the Langmuir model very well.
中文翻译:

氧化石墨烯、活性炭和多壁碳纳米管从水中吸附去除柔红霉素:平衡和动力学研究
研究了碳质吸附剂对水溶液中抗癌药物柔红霉素的吸附。比较了氧化石墨烯 (GO)、活性炭 (AC) 和多壁碳纳米管 (MWCNT) 的吸附性能。探讨了溶液 pH 值、接触时间和柔红霉素初始浓度对柔红霉素去除的影响。应用等温线和动力学模型来预测吸附剂的效率。吸附剂的主要官能团通过 FTIR 光谱法鉴定。在碱性介质中,吸附随pH值的增加而增加。随着初始浓度的增加,吸附容量增加,去除效率降低。动力学研究表明,伪二级模型很好地描述了对 GO 的吸附,而粒子内扩散模型最适合 AC 和 MWCNT。吸附等温线非常符合 Langmuir 模型。
更新日期:2022-09-20
中文翻译:

氧化石墨烯、活性炭和多壁碳纳米管从水中吸附去除柔红霉素:平衡和动力学研究
研究了碳质吸附剂对水溶液中抗癌药物柔红霉素的吸附。比较了氧化石墨烯 (GO)、活性炭 (AC) 和多壁碳纳米管 (MWCNT) 的吸附性能。探讨了溶液 pH 值、接触时间和柔红霉素初始浓度对柔红霉素去除的影响。应用等温线和动力学模型来预测吸附剂的效率。吸附剂的主要官能团通过 FTIR 光谱法鉴定。在碱性介质中,吸附随pH值的增加而增加。随着初始浓度的增加,吸附容量增加,去除效率降低。动力学研究表明,伪二级模型很好地描述了对 GO 的吸附,而粒子内扩散模型最适合 AC 和 MWCNT。吸附等温线非常符合 Langmuir 模型。


























 京公网安备 11010802027423号
京公网安备 11010802027423号