Journal of Advanced Research ( IF 10.7 ) Pub Date : 2022-09-18 , DOI: 10.1016/j.jare.2022.09.005 Kyung Min Lim 1 , Sehee Kim 2 , Jeonghun Yeom 3 , Yujin Choi 2 , Yoonjoo Lee 2 , Jongyub An 2 , Minchan Gil 2 , Ahmed Abdal Dayem 2 , Kyeongseok Kim 2 , Geun-Ho Kang 1 , Aram Kim 4 , Kwonho Hong 2 , Kyunggon Kim 5 , Ssang-Goo Cho 1
|
Introduction
Mesenchymal stromal cells (MSCs) release extracellular vesicles (MSC-EVs) containing various cargoes. Although MSC-EVs show significant therapeutic effects, the low production of EVs in MSCs hinders MSC-EV-mediated therapeutic development.
Objectives
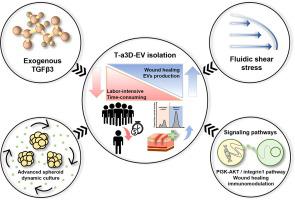
Here, we developed an advanced three-dimensional (a3D) dynamic culture technique with exogenous transforming growth factor beta-3 (TGF-β3) treatment (T-a3D) to produce potent MSC-EVs.
Methods
Our system enabled preparation of a highly concentrated EV-containing medium for efficient EV isolation and purification with higher yield and efficacy.
Results
MSC spheroids in T-a3D system (T-a3D spheroids) showed high expression of CD9 and TGF-β3, which was dependent on TGF-β signaling. Treatment with EVs produced under T-a3D conditions (T-a3D-EVs) led to significantly improved migration of dermal fibroblasts and wound closure in an excisional wound model. The relative total efficacy (relative yield of single-batch EVs (10–11-fold) × relative regeneration effect of EVs (2–3-fold)) of T-a3D-EVs was approximately up to 33-fold higher than that of 2D-EVs. Importantly the quantitative proteomic analyses of the T-a3D spheroids and T-a3D-EVs supported the improved EV production as well as the therapeutic potency of T-a3D-EVs.
Conclusion
TGF-β signalling differentially regulated by fluid shear stress produced in our system and exogenous TGF-β3 addition was confirmed to play an important role in the enhanced production of EVs with modified protein cargoes. We suggest that the T-a3D system leads to the efficient production of MSC-EVs with high potential in therapies and clinical development.
中文翻译:

具有转化生长因子-β3 的先进 3D 动态培养系统通过上调 TGF-β 信号传导增强具有修饰蛋白货物的有效细胞外囊泡的产生
介绍
间充质基质细胞 (MSC) 释放含有各种货物的细胞外囊泡 (MSC-EV)。尽管 MSC-EV 显示出显着的治疗效果,但 MSC 中 EV 的低产量阻碍了 MSC-EV 介导的治疗发展。
目标
在这里,我们开发了一种先进的三维 (a3D) 动态培养技术,采用外源转化生长因子 beta-3 (TGF-β3) 处理 (T-a3D) 来产生有效的 MSC-EV。
方法
我们的系统能够制备高度浓缩的含有 EV 的培养基,以实现高效的 EV 分离和纯化,并具有更高的产量和功效。
结果
T-a3D 系统(T-a3D 球体)中的 MSC 球体显示出 CD9 和 TGF-β3 的高表达,这依赖于 TGF-β 信号传导。用在 T-a3D 条件下产生的 EV (T-a3D-EV) 进行治疗可显着改善皮肤成纤维细胞的迁移和切除伤口模型中的伤口闭合。T-a3D-EV 的相对总功效(单批次 EV 的相对产量(10-11 倍)× EV 的相对再生效果(2-3 倍))比二维电动汽车。重要的是,T-a3D 球体和 T-a3D-EV 的定量蛋白质组学分析支持改进的 EV 生产以及 T-a3D-EV 的治疗效力。
结论
TGF-β 信号受我们系统中产生的流体剪切应力的差异调节,外源性 TGF-β3 添加被证实在增强含有修饰蛋白货物的 EV 生产中发挥重要作用。我们认为 T-a3D 系统可以高效生产 MSC-EV,在治疗和临床开发方面具有很高的潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号