当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Alkali metal-mediated interfacial charge redistribution toward near-optimal water oxidation
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2022-09-20 , DOI: 10.1039/d2ta04736e Ungsoo Kim 1 , Sangjin Lee 2 , Nam Khen Oh 1 , Jihyung Seo 1 , Ji Hoo Cha 1 , Junghyun Lee 1 , Seong-hun Lee 3 , Tae Joo Shin 3, 4 , Jeong Min Baik 5, 6 , Young-Kyu Han 2 , Hyesung Park 1, 4
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2022-09-20 , DOI: 10.1039/d2ta04736e Ungsoo Kim 1 , Sangjin Lee 2 , Nam Khen Oh 1 , Jihyung Seo 1 , Ji Hoo Cha 1 , Junghyun Lee 1 , Seong-hun Lee 3 , Tae Joo Shin 3, 4 , Jeong Min Baik 5, 6 , Young-Kyu Han 2 , Hyesung Park 1, 4
Affiliation
|
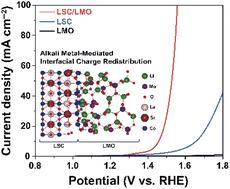
The optimal oxidation state and electronic structure of active sites in an electrocatalyst are critical factors for maximizing water-oxidation kinetics. To this end, we developed a heterostructured electrocatalyst for oxygen evolution reaction (OER) comprising La0.5Sr0.5CoO3−δ and Li2MoO4 (LSC/LMO) with optimized oxidation states for active metal sites using an alkali metal mediator. The LSC/LMO system exhibited excellent OER performance (overpotential: 1.45 V at 10 mA cm−2) and operational durability (chronoamperometric and cyclic voltammetry stabilities of 200 h at 1.52 V and 5000 cycles). The experimental and computational analyses revealed that lithium atoms accumulated at the LSC/LMO interface exhibit a mediating function toward optimizing the oxidation state and electronic structure of OER active metal elements (cobalt and molybdenum), minimizing the free energy barrier of the rate-determining step in OER. This study provides a new insight for boosting sluggish OER kinetics in water oxidation through in situ oxidation state modulation for heterostructured electrocatalysts.
中文翻译:

碱金属介导的界面电荷再分配向近乎最佳的水氧化
电催化剂中活性位点的最佳氧化态和电子结构是最大化水氧化动力学的关键因素。为此,我们开发了一种用于析氧反应 (OER) 的异质结构电催化剂,包含 La 0.5 Sr 0.5 CoO 3- δ和 Li 2 MoO 4 (LSC/LMO),使用碱金属介质对活性金属位点具有优化的氧化态。LSC/LMO 系统表现出优异的 OER 性能(过电位:1.45 V at 10 mA cm -2) 和操作耐久性(计时电流和循环伏安稳定性在 1.52 V 和 5000 次循环下为 200 小时)。实验和计算分析表明,在 LSC/LMO 界面上积累的锂原子对优化 OER 活性金属元素(钴和钼)的氧化态和电子结构具有调节作用,最大限度地降低了速率决定步骤的自由能垒在 OER 中。这项研究为通过异质结构电催化剂的原位氧化态调制来提高水氧化中缓慢的 OER 动力学提供了新的见解。
更新日期:2022-09-20
中文翻译:

碱金属介导的界面电荷再分配向近乎最佳的水氧化
电催化剂中活性位点的最佳氧化态和电子结构是最大化水氧化动力学的关键因素。为此,我们开发了一种用于析氧反应 (OER) 的异质结构电催化剂,包含 La 0.5 Sr 0.5 CoO 3- δ和 Li 2 MoO 4 (LSC/LMO),使用碱金属介质对活性金属位点具有优化的氧化态。LSC/LMO 系统表现出优异的 OER 性能(过电位:1.45 V at 10 mA cm -2) 和操作耐久性(计时电流和循环伏安稳定性在 1.52 V 和 5000 次循环下为 200 小时)。实验和计算分析表明,在 LSC/LMO 界面上积累的锂原子对优化 OER 活性金属元素(钴和钼)的氧化态和电子结构具有调节作用,最大限度地降低了速率决定步骤的自由能垒在 OER 中。这项研究为通过异质结构电催化剂的原位氧化态调制来提高水氧化中缓慢的 OER 动力学提供了新的见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号