Biomedicine & Pharmacotherapy ( IF 7.5 ) Pub Date : 2022-09-19 , DOI: 10.1016/j.biopha.2022.113695 G D Marijn Veerman 1 , Daan P Hurkmans 1 , Marthe S Paats 2 , Esther Oomen-de Hoop 1 , Cor H van der Leest 3 , Eric R E van Thiel 4 , Joachim G J V Aerts 2 , Roelof W van Leeuwen 5 , Anne-Marie C Dingemans 2 , Ron H J Mathijssen 1
|
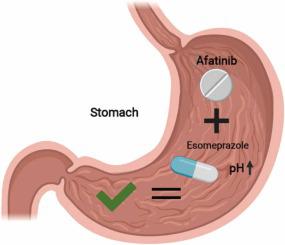
Afatinib is an oral small-molecule kinase inhibitor (SMKI) approved for treatment of metastatic non-small cell lung cancer (NSCLC) with an epidermal growth factor receptor (EGFR) driver mutation. Although oral administration is convenient, most SMKIs experience pH-dependent solubility. A drug-drug interaction between afatinib and proton-pump inhibitors (PPIs) has, however, never been studied in humans. Hence, we performed a randomized, three-period cross-over study. Afatinib (30 mg or 40 mg) was administered without PPI (period A), concomitantly with esomeprazole (period B) and three hours after esomeprazole intake (period C). Primary objective was the area under the curve (AUC0–24 h) comparing period A to period B and period A to period C. Secondary objectives were other pharmacokinetic parameters and toxicity. Linear mixed effect modelling was performed for differences in AUC0–24 h and Cmax between periods A and B and periods A and C. In 18 evaluable NSCLC patients, concomitant use of 40 mg esomeprazole decreased the steady-state afatinib AUC0–24 h with 10.2% (95% CI −29.2 to +14.0%; p = 0.564) compared to afatinib administration without PPI. Esomeprazole intake three hours prior to afatinib did not significantly influence afatinib AUC0–24 h (−0.6%; 95% CI −14.9 to +16.1%; p = 1.0). No differences in toxicity were observed. To conclude, esomeprazole did not change the exposure to afatinib in patients with NSCLC. Since there is no clinically relevant drug-drug interaction, esomeprazole can safely be co-administered with afatinib. This is important for clinical practice, because other EGFR-SMKIs (e.g. erlotinib and gefitinib) do experience clinically relevant drug-drug interactions with acid-suppressive agents.
中文翻译:

埃索美拉唑对阿法替尼生物利用度的影响:非小细胞肺癌患者的药代动力学交叉研究
阿法替尼是一种口服小分子激酶抑制剂(SMKI),被批准用于治疗具有表皮生长因子受体(EGFR)驱动突变的转移性非小细胞肺癌(NSCLC)。尽管口服给药很方便,但大多数 SMKI 的溶解度取决于 pH 值。然而,从未在人体中研究过阿法替尼和质子泵抑制剂 (PPI) 之间的药物相互作用。因此,我们进行了一项随机的三期交叉研究。阿法替尼(30 mg 或 40 mg)在没有 PPI(A 期)的情况下给药,同时与埃索美拉唑(B 期)和埃索美拉唑摄入后 3 小时(C 期)给药。主要目标是曲线下面积(AUC 0-24 h) 将 A 期与 B 期以及 A 期与 C 期进行比较。次要目标是其他药代动力学参数和毒性。对 A 期和 B 期以及 A 期和 C 期之间 AUC 0-24 h和 C max的差异进行线性混合效应建模。在 18 名可评估的 NSCLC 患者中,同时使用 40 mg 埃索美拉唑降低了稳态阿法替尼 AUC 0-24与没有 PPI 的阿法替尼给药相比, h为 10.2%(95% CI -29.2 至 +14.0%;p = 0.564)。阿法替尼前 3 小时服用埃索美拉唑对0-24 小时阿法替尼 AUC 无显着影响(-0.6%;95% CI -14.9 至 +16.1%;p = 1.0)。未观察到毒性差异。总而言之,埃索美拉唑并未改变 NSCLC 患者对阿法替尼的暴露。由于没有临床相关的药物-药物相互作用,埃索美拉唑可以安全地与阿法替尼联合给药。这对临床实践很重要,因为其他 EGFR-SMKI(例如厄洛替尼和吉非替尼)确实会与酸抑制剂发生临床相关的药物-药物相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号